The 5'-terminal stem-loop RNA element of SARS-CoV-2 features highly dynamic structural elements that are sensitive to differences in cellular pH
- PMID: 38842942
- PMCID: PMC11260494
- DOI: 10.1093/nar/gkae477
The 5'-terminal stem-loop RNA element of SARS-CoV-2 features highly dynamic structural elements that are sensitive to differences in cellular pH
Abstract
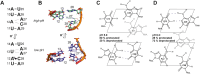
We present the nuclear magnetic resonance spectroscopy (NMR) solution structure of the 5'-terminal stem loop 5_SL1 (SL1) of the SARS-CoV-2 genome. SL1 contains two A-form helical elements and two regions with non-canonical structure, namely an apical pyrimidine-rich loop and an asymmetric internal loop with one and two nucleotides at the 5'- and 3'-terminal part of the sequence, respectively. The conformational ensemble representing the averaged solution structure of SL1 was validated using NMR residual dipolar coupling (RDC) and small-angle X-ray scattering (SAXS) data. We show that the internal loop is the major binding site for fragments of low molecular weight. This internal loop of SL1 can be stabilized by an A12-C28 interaction that promotes the transient formation of an A+•C base pair. As a consequence, the pKa of the internal loop adenosine A12 is shifted to 5.8, compared to a pKa of 3.63 of free adenosine. Furthermore, applying a recently developed pH-differential mutational profiling (PD-MaP) approach, we not only recapitulated our NMR findings of SL1 but also unveiled multiple sites potentially sensitive to pH across the 5'-UTR of SARS-CoV-2.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






Similar articles
-
1H, 13C and 15N assignment of stem-loop SL1 from the 5'-UTR of SARS-CoV-2.Biomol NMR Assign. 2021 Oct;15(2):467-474. doi: 10.1007/s12104-021-10047-2. Epub 2021 Aug 28. Biomol NMR Assign. 2021. PMID: 34453696 Free PMC article.
-
Structure of an internal loop motif with three consecutive U•U mismatches from stem-loop 1 in the 3'-UTR of the SARS-CoV-2 genomic RNA.Nucleic Acids Res. 2024 Jun 24;52(11):6687-6706. doi: 10.1093/nar/gkae349. Nucleic Acids Res. 2024. PMID: 38783391 Free PMC article.
-
High frequency of transition to transversion ratio in the stem region of RNA secondary structure of untranslated region of SARS-CoV-2.PeerJ. 2024 Apr 22;12:e16962. doi: 10.7717/peerj.16962. eCollection 2024. PeerJ. 2024. PMID: 38666080 Free PMC article.
-
Untangling the pseudoknots of SARS-CoV-2: Insights into structural heterogeneity and plasticity.Curr Opin Struct Biol. 2024 Oct;88:102912. doi: 10.1016/j.sbi.2024.102912. Epub 2024 Aug 21. Curr Opin Struct Biol. 2024. PMID: 39168046 Review.
-
Hepatitis B virus nuclear export elements: RNA stem-loop α and β, key parts of the HBV post-transcriptional regulatory element.RNA Biol. 2016 Sep;13(9):743-7. doi: 10.1080/15476286.2016.1166330. Epub 2016 Mar 31. RNA Biol. 2016. PMID: 27031749 Free PMC article. Review.
Cited by
-
Targeting the SARS-CoV-2 RNA Translation Initiation Element SL1 by Molecules of Low Molecular Weight.J Am Chem Soc. 2025 Aug 13;147(32):28783-28798. doi: 10.1021/jacs.5c05264. Epub 2025 Aug 4. J Am Chem Soc. 2025. PMID: 40758647 Free PMC article.
-
Dissecting the Conformational Heterogeneity of Stem-Loop Substructures of the Fifth Element in the 5'-Untranslated Region of SARS-CoV-2.J Am Chem Soc. 2024 Nov 6;146(44):30139-30154. doi: 10.1021/jacs.4c08406. Epub 2024 Oct 23. J Am Chem Soc. 2024. PMID: 39442924 Free PMC article.
-
NMR characterisation of the antibiotic resistance-mediating 32mer RNA from the 23S ribosomal RNA.Biomol NMR Assign. 2025 Jun;19(1):133-145. doi: 10.1007/s12104-025-10229-2. Epub 2025 Apr 3. Biomol NMR Assign. 2025. PMID: 40175819 Free PMC article.
-
Insights into the A-C Mismatch Conformational Ensemble in Duplex DNA and its Role in Genetic Processes through a Structure-based Review.J Mol Biol. 2024 Sep 15;436(18):168710. doi: 10.1016/j.jmb.2024.168710. Epub 2024 Jul 14. J Mol Biol. 2024. PMID: 39009073 Review.
References
-
- Schubert K., Karousis E.D., Jomaa A., Scaiola A., Echeverria B., Gurzeler L.A., Leibundgut M., Thiel V., Mühlemann O., Ban N. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat. Struct. Mol. Biol. 2020; 27:959–966. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

