Modeling transthyretin (TTR) amyloid diseases, from monomer to amyloid fibrils
- PMID: 38843135
- PMCID: PMC11156392
- DOI: 10.1371/journal.pone.0304891
Modeling transthyretin (TTR) amyloid diseases, from monomer to amyloid fibrils
Abstract
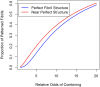
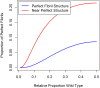
ATTR amyloidosis is caused by deposition of large, insoluble aggregates (amyloid fibrils) of cross-β-sheet TTR protein molecules on the intercellular surfaces of tissues. The process of amyloid formation from monomeric TTR protein molecules to amyloid deposits has not been fully characterized and is therefore modeled in this paper. Two models are considered: 1) TTR monomers in the blood spontaneously fold into a β-sheet conformation, aggregate into short proto-fibrils that then circulate in the blood until they find a complementary tissue where the proto-fibrils accumulate to form the large, insoluble amyloid fibrils found in affected tissues. 2) TTR monomers in the native or β-sheet conformation circulate in the blood until they find a tissue binding site and deposit in the tissue or tissues forming amyloid deposits in situ. These models only differ on where the selection for β-sheet complementarity occurs, in the blood where wt-wt, wt-v, and v-v interactions determine selectivity, or on the tissue surface where tissue-wt and tissure-v interactions also determine selectivity. Statistical modeling in both cases thus involves selectivity in fibril aggregation and tissue binding. Because binding of protein molecules into fibrils and binding of fibrils to tissues occurs through multiple weak non-covalent bonds, strong complementarity between β-sheet molecules and between fibrils and tissues is required to explain the insolubility and tissue selectivity of ATTR amyloidosis. Observation of differing tissue selectivity and thence disease phenotypes from either pure wildtype TTR protein or a mix of wildtype and variant molecules in amyloid fibrils evidences the requirement for fibril-tissue complementarity. Understanding the process that forms fibrils and binds fibrils to tissues may lead to new possibilities for interrupting the process and preventing or curing ATTR amyloidosis.
Copyright: © 2024 Criddle et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Amyloid seeding of transthyretin by ex vivo cardiac fibrils and its inhibition.Proc Natl Acad Sci U S A. 2018 Jul 17;115(29):E6741-E6750. doi: 10.1073/pnas.1805131115. Epub 2018 Jun 28. Proc Natl Acad Sci U S A. 2018. PMID: 29954863 Free PMC article.
-
Tolcapone, a potent aggregation inhibitor for the treatment of familial leptomeningeal amyloidosis.FEBS J. 2021 Jan;288(1):310-324. doi: 10.1111/febs.15339. Epub 2020 May 11. FEBS J. 2021. PMID: 32324953
-
Cryo-EM structure of a transthyretin-derived amyloid fibril from a patient with hereditary ATTR amyloidosis.Nat Commun. 2019 Nov 1;10(1):5008. doi: 10.1038/s41467-019-13038-z. Nat Commun. 2019. PMID: 31676763 Free PMC article.
-
A Brief Journey through Protein Misfolding in Transthyretin Amyloidosis (ATTR Amyloidosis).Int J Mol Sci. 2021 Dec 6;22(23):13158. doi: 10.3390/ijms222313158. Int J Mol Sci. 2021. PMID: 34884963 Free PMC article. Review.
-
Mechanisms of transthyretin aggregation and toxicity.Subcell Biochem. 2012;65:211-24. doi: 10.1007/978-94-007-5416-4_9. Subcell Biochem. 2012. PMID: 23225005 Review.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

