Repair of the Infarcted Heart: Cellular Effectors, Molecular Mechanisms and Therapeutic Opportunities
- PMID: 38843294
- PMCID: PMC11164543
- DOI: 10.1161/CIRCRESAHA.124.323658
Repair of the Infarcted Heart: Cellular Effectors, Molecular Mechanisms and Therapeutic Opportunities
Abstract
The adult mammalian heart has limited endogenous regenerative capacity and heals through the activation of inflammatory and fibrogenic cascades that ultimately result in the formation of a scar. After infarction, massive cardiomyocyte death releases a broad range of damage-associated molecular patterns that initiate both myocardial and systemic inflammatory responses. TLRs (toll-like receptors) and NLRs (NOD-like receptors) recognize damage-associated molecular patterns (DAMPs) and transduce downstream proinflammatory signals, leading to upregulation of cytokines (such as interleukin-1, TNF-α [tumor necrosis factor-α], and interleukin-6) and chemokines (such as CCL2 [CC chemokine ligand 2]) and recruitment of neutrophils, monocytes, and lymphocytes. Expansion and diversification of cardiac macrophages in the infarcted heart play a major role in the clearance of the infarct from dead cells and the subsequent stimulation of reparative pathways. Efferocytosis triggers the induction and release of anti-inflammatory mediators that restrain the inflammatory reaction and set the stage for the activation of reparative fibroblasts and vascular cells. Growth factor-mediated pathways, neurohumoral cascades, and matricellular proteins deposited in the provisional matrix stimulate fibroblast activation and proliferation and myofibroblast conversion. Deposition of a well-organized collagen-based extracellular matrix network protects the heart from catastrophic rupture and attenuates ventricular dilation. Scar maturation requires stimulation of endogenous signals that inhibit fibroblast activity and prevent excessive fibrosis. Moreover, in the mature scar, infarct neovessels acquire a mural cell coat that contributes to the stabilization of the microvascular network. Excessive, prolonged, or dysregulated inflammatory or fibrogenic cascades accentuate adverse remodeling and dysfunction. Moreover, inflammatory leukocytes and fibroblasts can contribute to arrhythmogenesis. Inflammatory and fibrogenic pathways may be promising therapeutic targets to attenuate heart failure progression and inhibit arrhythmia generation in patients surviving myocardial infarction.
Keywords: angiogenesis; fibroblasts; fibrosis; infarction; inflammation; macrophages.
Conflict of interest statement
Figures

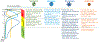
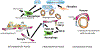
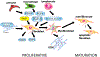
References
-
- Thrane PG, Olesen KKW, Thim T, Gyldenkerne C, Mortensen MB, Kristensen SD, Maeng M. Mortality Trends After Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2023; 82:999–1010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

