Sacituzumab Govitecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non-Small Cell Lung Cancer: The Randomized, Open-Label Phase III EVOKE-01 Study
- PMID: 38843511
- PMCID: PMC11328920
- DOI: 10.1200/JCO.24.00733
Sacituzumab Govitecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non-Small Cell Lung Cancer: The Randomized, Open-Label Phase III EVOKE-01 Study
Abstract
Purpose: The open-label, phase III EVOKE-01 study evaluated sacituzumab govitecan (SG) versus standard-of-care docetaxel in metastatic non-small cell lung cancer (mNSCLC) with progression on/after platinum-based chemotherapy, anti-PD-(L)1, and targeted treatment for actionable genomic alterations (AGAs). Primary analysis is reported.
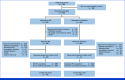
Methods: Patients were randomly assigned 1:1 (stratified by histology, best response to last anti-PD-(L)1-containing regimen, and AGA treatment received or not) to SG (one 10 mg/kg intravenous infusion on days 1 and 8) or docetaxel (one 75 mg/m2 intravenous infusion on day 1) in 21-day cycles. Primary end point was overall survival (OS). Key secondary end points were investigator-assessed progression-free survival (PFS), objective response rate, patient-reported symptom assessment, and safety.
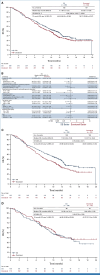
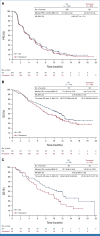
Results: In the intention-to-treat population (SG, n = 299; docetaxel, n = 304), 55.4% had one previous line of therapy. Median follow-up was 12.7 months (range, 6.0-24.0). The primary end point was not met. There was a numerical OS improvement for SG versus docetaxel (median, 11.1 v 9.8 months; hazard ratio [HR], 0.84 [95% CI, 0.68 to 1.04]; one-sided P = .0534), consistent across squamous and nonsquamous histologies. Median PFS was 4.1 versus 3.9 months (HR, 0.92 [95% CI, 0.77 to 1.11]). An OS benefit was observed for SG (n = 192) versus docetaxel (n = 191) in mNSCLC nonresponsive to last anti-PD-(L)1-containing regimen (3.5-month median OS increase; HR, 0.75 [95% CI, 0.58 to 0.97]); this was consistent across histologies. Among patients receiving SG and docetaxel, 6.8% and 14.2% discontinued because of treatment-related adverse events (TRAEs), respectively; 1.4% and 1.0%, respectively, had TRAEs leading to death.
Conclusion: Although statistical significance was not met, OS numerically improved with SG versus docetaxel, which was consistent across histologies. Clinically meaningful improvement in OS was noted in mNSCLC nonresponsive to last anti-PD-(L)1-containing regimen. SG was better tolerated than docetaxel and consistent with its known safety profile, with no new safety signals.
Trial registration: ClinicalTrials.gov NCT05089734.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Ettinger DS, Wood DE, Aisner DL, et al. : NCCN Guidelines® insights: Non-small cell lung cancer, version 2.2023. J Natl Compr Canc Netw 21:340-350, 2023 - PubMed
-
- Borghaei H, de Marinis F, Dumoulin D, et al. : SAPPHIRE: Phase III study of sitravatinib plus nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. Ann Oncol 35:66-76, 2024 - PubMed
-
- Neal J, Pavlakis N, Kim S-W, et al. : CONTACT-01: Efficacy and safety from a phase III study of atezolizumab (atezo) + cabozantinib (cabo) vs docetaxel (doc) monotherapy in patients (pts) with metastatic NSCLC (mNSCLC) previously treated with checkpoint inhibitors and chemotherapy. J Thorac Oncol 41:S39-S40, 2023
-
- Paz-Ares L, Goto Y, Lim WDT, et al. : 1194MO canakinumab (CAN) + docetaxel (DTX) for the second- or third-line (2/3L) treatment of advanced non-small cell lung cancer (NSCLC): CANOPY-2 phase III results. Ann Oncol 32:S953-S954, 2021
-
- Ahn M, Lisberg AE, Paz-Ares L, et al. : Datopotamab deruxtecan (Dato-DXd) vs docetaxel in previously treated advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC): Results of the randomized phase III study TROPION-Lung01. Ann Oncol 34:S1661-S1706, 2023
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

