Response-adapted ultra-low-dose 4 Gy radiation as definitive therapy of gastric MALT lymphoma: a single-centre, pilot trial
- PMID: 38843856
- PMCID: PMC11211047
- DOI: 10.1016/S2352-3026(24)00133-9
Response-adapted ultra-low-dose 4 Gy radiation as definitive therapy of gastric MALT lymphoma: a single-centre, pilot trial
Erratum in
-
Correction to Lancet Haematol 2024; 11: e521-29.Lancet Haematol. 2025 Jul;12(7):e489. doi: 10.1016/S2352-3026(25)00174-7. Lancet Haematol. 2025. PMID: 40610171 No abstract available.
Abstract
Background: Given the favourable prognosis of patients with gastric mucosa-associated lymphoid tissue (MALT) lymphoma, treatment-related toxicity should be minimised. We aimed to evaluate the efficacy of 4 Gy radiotherapy given in a response-adapted approach.
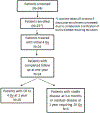
Methods: We conducted a single-centre, single-arm, prospective trial at MD Anderson Cancer Center (Houston, TX, USA) of response-adapted ultra-low-dose radiotherapy. Eligible patients were 18 years or older and had newly diagnosed or relapsed Helicobacter pylori-negative gastric MALT lymphoma, with stage I-IV disease. Given the expected low toxicity profile of treatment, performance status was not an exclusion criterion. Patients received external beam photon-based radiotherapy for a total dose of 4 Gy in two fractions. Patients with a complete response to 4 Gy via endoscopy and imaging at 3-4 months were observed; patients with a partial response were re-evaluated in 6-9 months. Residual disease at 9-13 months or stable or progressive disease at any time required additional treatment with 20 Gy. The primary endpoint was gastric complete response at 1 year (second evaluation timepoint) after 4 Gy treatment. All analyses were performed as intention to treat. This trial is registered at ClinicalTrials.gov (NCT03680586) and is complete and closed to enrolment.
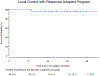
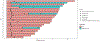
Findings: Between March 27, 2019, and Oct 12, 2021, we enrolled 24 eligible patients. The median age of participants was 67 years (IQR 58-74; range 40-85); 15 (63%) were female and nine (37%) male; 18 (75%) were White, four (17%) Asian, and two (8%) Hispanic; 20 (83%) had stage I disease, one (4%) stage II, and three (13%) stage IV. Median follow-up time was 36 months (IQR 26-42). 20 patients (83%) had a complete response to 4 Gy (16 at 3-4 months, four at 9-13 months); two patients received 20 Gy for symptomatic stable disease at 3-4 months and two for residual disease at 9-13 months; all had a complete response. The 3-year local control rate was 96% (95% CI 88-100), with one local relapse at 14 months after 4 Gy radiotherapy salvaged successfully with 20 Gy. One patient with stage IV disease had a distant relapse. The most common adverse events were grade 1 nausea (nine [38%] of 24 patients who received 4 Gy and two [50%] of four patients who received 20 Gy) and grade 1 abdominal pain (five [21%] of 24 and zero of four, respectively). No grade 3 or worse adverse events were noted, including no treatment-related deaths.
Interpretation: Most patients had a complete response after 4 Gy radiotherapy; all who required an additional 20 Gy had a complete response within 12 months. This response-adapted strategy could be used to select patients who would benefit from additional radiotherapy and spare others potential associated toxicity.
Funding: National Cancer Institute.
Copyright © 2024 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declarations of interests CCP has received research support from Merck. All other authors declare no competing interests.
Figures

References
-
- Schmelz R, Miehlke S, Thiede C, et al. Sequential H. pylori eradication and radiation therapy with reduced dose compared to standard dose for gastric MALT lymphoma stages IE & II1E: a prospective randomized trial. Journal of gastroenterology 2019; 54(5): 388–95. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

