Vimseltinib versus placebo for tenosynovial giant cell tumour (MOTION): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial
- PMID: 38843860
- PMCID: PMC11740396
- DOI: 10.1016/S0140-6736(24)00885-7
Vimseltinib versus placebo for tenosynovial giant cell tumour (MOTION): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial
Abstract
Background: Tenosynovial giant cell tumour (TGCT) is a locally aggressive neoplasm for which few systemic treatment options exist. This study evaluated the efficacy and safety of vimseltinib, an oral, switch-control, CSF1R inhibitor, in patients with symptomatic TGCT not amenable to surgery.
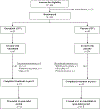
Methods: MOTION is a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial done in 35 specialised hospitals in 13 countries. Eligible patients were adults (aged ≥18 years) with a histologically confirmed diagnosis of TGCT for which surgical resection could potentially worsen functional limitation or cause severe morbidity. Patients were randomly assigned (2:1) with interactive response technology to vimseltinib (30 mg orally twice weekly) or placebo, administrated in 28-day cycles for 24 weeks. Patients and site personnel were masked to treatment assignment until week 25, unless progressive disease was confirmed earlier. The primary endpoint was objective response rate by independent radiological review using Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST) at week 25 in the intention-to-treat population. Safety was assessed in all patients who received the study drug. The trial is registered with ClinicalTrials.gov, NCT05059262, and enrolment is complete.
Findings: Between Jan 21, 2022, and Feb 21, 2023, 123 patients were randomly assigned (83 to vimseltinib and 40 to placebo). 73 (59%) patients were female and 50 (41%) were male. Nine (11%) of 83 patients assigned to vimseltinib and five (13%) of 40 patients assigned to placebo discontinued treatment before week 25; one patient in the placebo group did not receive any study drug. Objective response rate per RECIST was 40% (33 of 83 patients) in the vimseltinib group vs 0% (none of 40) in the placebo group (difference 40% [95% CI 29-51]; p<0·0001). Most treatment-emergent adverse events (TEAEs) were grade 1 or 2; the only grade 3 or 4 TEAE that occurred in more than 5% of patients receiving vimseltinib was increased blood creatine phosphokinase (eight [10%] of 83). One patient in the vimseltinib group had a treatment-related serious TEAE of subcutaneous abscess. No evidence of cholestatic hepatotoxicity or drug-induced liver injury was noted.
Interpretation: Vimseltinib produced a significant objective response rate and clinically meaningful functional and symptomatic improvement in patients with TGCT, providing an effective treatment option for these patients.
Funding: Deciphera Pharmaceuticals.
Copyright © 2024 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests VB reports institutional research funding from Deciphera Pharmaceuticals and participation on a medical advisory board for Deciphera Pharmaceuticals. SS reports institutional research funding from Abbiski, Daiichi Sankyo, and Deciphera Pharmaceuticals, and advisory board roles with Daiichi Sankyo and Deciphera Pharmaceuticals. SB reports honoraria from Blueprint Medicine, Boehringer Ingelheim, Pfizer, PharmaMar, Uptodate, and Deciphera Pharmaceuticals; consulting or advisory roles with Adcendo, Bayer, Boehringer Ingelheim, Cogent Biosciences, Daiichi Sankyo, Lilly, IDRx, and Deciphera Pharmaceuticals; institutional research funding from Adcendo, Blueprint Medicine, Incyte, and IDRx; participation on a board for the University of Aachen; unpaid board of directors positions with The Connective Tissue Oncology Society and Deutsche Sarkomstiftung; and a faculty position with the European Society for Medical Oncology. AJW reports consulting or advisory roles and institutional research funding from Daiichi Sankyo and Deciphera Pharmaceuticals. MvdS reports travel partly supported by Deciphera Pharmaceuticals. NMB reports research support from Deciphera Pharmaceuticals. AAR reports consulting or advisory roles with Boehringer Ingelheim and Medison; institutional research funding from 23andMe, Abbisko, AbbVie, Adaptimmune, Amgen, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, GSK, Inhibrx, Iterion Therapeutics, Karyopharm Therapeutics, MedImmune, Medison, Merck, Neoleukin Therapeutics, Pfizer, Rain Therapeutics, Roche/Genentech, Symphogen, and Deciphera Pharmaceuticals; and participation on an advisory board for Inhibrx. AI reports consulting or advisory roles for Bayer, Bristol Myers Squibb, Chugai Pharmaceutical, Merck, Merck Sharp & Dohme, Novartis, Parthenon, PharmaMar, and Roche; and institutional research funding from Bayer, Bristol Myers Squibb, Chugai Pharmaceutical, Merck, Merck Sharp & Dohme, Novartis, Parthenon, PharmaMar, and Roche. ALC reports honoraria from Bayer, PharmaMar, and Deciphera Pharmaceuticals. GT reports consulting or advisory roles with Daiichi Sankyo and Servier. KB reports honoraria from Incyte, Lilly, Novartis, and Deciphera Pharmaceuticals; expert testimony for Deciphera Pharmaceuticals; and advisory board participation for Bayer, GSK, and NEC OncoImmunity. JM-B reports consulting or advisory roles for Amgen, Asofarma, Boehringer Ingelheim, Bayer, GSK, Lilly, Novartis, PharmaMar, Roche, and Tecnofarma; speakers bureau involvement for PharmaMar; and institutional research funding from Adaptimmune, Amgen, Ayala Pharmaceuticals, Bayer, Blueprint Medicines, Bristol Myers Squibb, Cebiotex, Celgene, Daiichi Sankyo, Eisai, GSK, IMMIX Biopharma, Inhibrx, Karyopharm Therapeutics, Lilly, Lixte, Novartis, Pfizer, PharmaMar, Philogen, PTC Therapeutics, Rain Therapeutics, and Deciphera Pharmaceuticals; expert testimony for Amgen, Bayer, Boehringer Ingelheim, Eisai, Lilly, PharmaMar, and Roche; travel support from Novartis, Pfizer, and PharMar; advisory board participation for Asofarma and Tecnofarma; and a leadership or fiduciary role for Sarcoma Research solutions. EP reports advisory board roles with Daiichi Sankyo, SynOx, and Deciphera Pharmaceuticals. PRe reports honoraria for participation in advisory boards for Bayer, Blueprint Medicines, Boehringer Ingelheim, Clinigen, GSK, MundiBioPharma, Novartis, PharmaMar, Roche, and Deciphera Pharmaceuticals, and a leadership position for the German Sarcoma Foundation. PRu reports honoraria from AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, Pierre Fabre, and Sanofi; speakers bureaus for Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, and Pierre Fabre; travel support from Orphan Europe and Pierre Fabre; consulting fees from Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, Philogen, and Pierre Fabre; and institutional research funding from Bristol Myers Squibb, Novartis, Pfizer, and Roche. CT, FZ, and BH report employment with and stock or other ownership interests in Deciphera Pharmaceuticals. MGS reports employment with, stock or other ownership interests in, and travel support from Deciphera Pharmaceuticals. RR-S reports employment with, stock or other ownership interests in, and patents, royalties, or other intellectual property from Deciphera Pharmaceuticals (inventor in pending patents at Deciphera Pharmaceuticals for which the rights have been transferred to Deciphera Pharmaceuticals; RR-S has not received and will not receive any royalties). MLS reports employment in a leadership role with, stock or other ownership interests in, travel support from, and patents, royalties, or other intellectual property from Deciphera Pharmaceuticals (inventor in pending patents at Deciphera Pharmaceuticals for which the rights have been transferred to Deciphera Pharmaceuticals; MLS has not received and will not receive any royalties). J-YB reports honoraria from Bayer and Deciphera Pharmaceuticals; consulting or advisory roles with Bayer and Deciphera Pharmaceuticals; institutional research funding from Bayer and Deciphera Pharmaceuticals; academic support from the French National Cancer Institute (INCA) NETSARC, INTERSARC, and EURACAN; and funding for travel, accommodation, or expenses from OSE Pharma. WDT reports advisory board roles for Aadi Biosciences, Abbisko, Amgen, AmMax Bio, Avacta, Bayer, BioAtla, Boehringer Ingelheim, C4 Therapeutics, Cogent Biosciences, Daiichi Sankyo, Foghorn Therapeutics, IMGT, Inhibrx, Ipsen, Lilly, PharmaEssentia, Servier, Sonata, and Deciphera Pharmaceuticals; owns stock or shares in Atropos and Certis Oncology Solutions; reports a patent for Companion Diagnostics for CDK4 inhibitors (14/854,329) and for Enigma and CDH18 (SKI2016–021–03); and reports institutional funding from Deciphera Pharmaceuticals. All other authors declare no competing interests.
Figures


Comment in
-
Vimseltinib for tenosynovial giant cell tumour.Lancet. 2024 Jun 22;403(10445):2665-2667. doi: 10.1016/S0140-6736(24)01113-9. Epub 2024 Jun 3. Lancet. 2024. PMID: 38843859 No abstract available.
References
-
- Stacchiotti S, Durr HR, Schaefer IM, et al. Best clinical management of tenosynovial giant cell tumour (TGCT): a consensus paper from the community of experts. Cancer Treat Rev 2023; 112: 102491. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

