Unlocking Transplant Tolerance with Biomaterials
- PMID: 38843866
- PMCID: PMC11834385
- DOI: 10.1002/adhm.202400965
Unlocking Transplant Tolerance with Biomaterials
Abstract
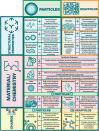
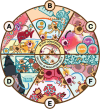
For patients suffering from organ failure due to injury or autoimmune disease, allogeneic organ transplantation with chronic immunosuppression is considered the god standard in terms of clinical treatment. However, the true "holy grail" of transplant immunology is operational tolerance, in which the recipient exhibits a sustained lack of alloreactivity toward unencountered antigen presented by the donor graft. This outcome is resultant from critical changes to the phenotype and genotype of the immune repertoire predicated by the activation of specific signaling pathways responsive to soluble and mechanosensitive cues. Biomaterials have emerged as a medium for interfacing with and reprogramming these endogenous pathways toward tolerance in precise, minimally invasive, and spatiotemporally defined manners. By viewing seminal and contemporary breakthroughs in transplant tolerance induction through the lens of biomaterials-mediated immunomodulation strategies-which include intrinsic material immunogenicity, the depot effect, graft coatings, induction and delivery of tolerogenic immune cells, biomimicry of tolerogenic immune cells, and in situ reprogramming-this review emphasizes the stunning diversity of approaches in the field and spotlights exciting future directions for research to come.
Keywords: biomaterials; immunoengineering; immunomodulation; transplantation.
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Fishman J. A., Rubin R. H., N. Engl. J. Med. 1998, 338, 1741. - PubMed
-
- Kato T., Gaynor J. J., Yoshida H., Montalvano M., Takahashi H., Pyrsopoulos N., Nishida S., Moon J., Selvaggi G., Levi D., Ruiz P., Schiff E., Tzakis A., Transplantation 2007, 84, 829. - PubMed
-
- Shapiro A. M. J., Lakey J. R. T., Ryan E. A., Korbutt G. S., Toth E., Warnock G. L., Kneteman N. M., Rajotte R. V., N. Engl. J. Med. 2000, 343, 230. - PubMed
-
- Shapiro A. M. J., Ricordi C., Hering B. J., Auchincloss H., Lindblad R., Robertson R. P., Secchi A., Brendel M. D., Berney T., Brennan D. C., Cagliero E., Alejandro R., Ryan E. A., DiMercurio B., Morel P., Polonsky K. S., Reems J.‐A., Bretzel R. G., Bertuzzi F., Froud T., Kandaswamy R., Sutherland D. E. R., Eisenbarth G., Segal M., Preiksaitis J., Korbutt G. S., Barton F. B., Viviano L., Seyfert‐Margolis V., Bluestone J., et al., N. Engl. J. Med. 2006, 355, 1318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

