A randomised phase II study of extended pleurectomy/decortication preceded or followed by chemotherapy in patients with early-stage pleural mesothelioma: EORTC 1205
- PMID: 38843916
- PMCID: PMC11211697
- DOI: 10.1183/13993003.02114-2023
A randomised phase II study of extended pleurectomy/decortication preceded or followed by chemotherapy in patients with early-stage pleural mesothelioma: EORTC 1205
Abstract
Background: The role of surgery in pleural mesothelioma remains controversial. It may be appropriate in highly selected patients as part of a multimodality treatment including chemotherapy. Recent years have seen a shift from extrapleural pleuropneumonectomy toward extended pleurectomy/decortication. The most optimal sequence of surgery and chemotherapy remains unknown.
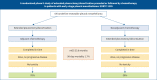
Methods: EORTC-1205-LCG was a multicentric, noncomparative phase 2 trial, 1:1 randomising between immediate (arm A) and deferred surgery (arm B), followed or preceded by chemotherapy. Eligible patients (Eastern Cooperative Oncology Group 0-1) had treatment-naïve, borderline resectable T1-3 N0-1 M0 mesothelioma of any histology. Primary outcome was rate of success at 20 weeks, a composite end-point including 1) successfully completing both treatments within 20 weeks; 2) being alive with no signs of progressive disease; and 3) no residual grade 3-4 toxicity. Secondary end-points were toxicity, overall survival, progression-free survival and process indicators of surgical quality.
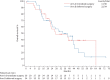
Findings: 69 patients were included in this trial. 56 (81%) patients completed three cycles of chemotherapy and 58 (84%) patients underwent surgery. Of the 64 patients in the primary analysis, 21 out of 30 patients in arm A (70.0%; 80% CI 56.8-81.0%) and 17 out of 34 patients (50.0%; 80% CI 37.8-62.2%) in arm B reached the statistical end-point for rate of success. Median progression-free survival and overall survival were 10.8 (95% CI 8.5-17.2) months and 27.1 (95% CI 22.6-64.3) months in arm A, and 8.0 (95% CI 7.2-21.9) months and 33.8 (95% CI 23.8-44.6) months in arm B. Macroscopic complete resection was obtained in 82.8% of patients. 30- and 90-day mortality were both 1.7%. No new safety signals were found, but treatment-related morbidity was high.
Interpretation: EORTC 1205 did not succeed in selecting a preferred sequence of pre- or post-operative chemotherapy. Either procedure is feasible with a low mortality, albeit consistent morbidity. A shared informed decision between surgeon and patient remains essential.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Figures


Comment in
-
Pleural mesothelioma: surgery questioned again?Eur Respir J. 2024 Jun 28;63(6):2400896. doi: 10.1183/13993003.00896-2024. Print 2024 Jun. Eur Respir J. 2024. PMID: 38942440 No abstract available.
References
-
- Treasure T, Lang-Lazdunski L, Waller D, et al. . Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011; 12: 763–772. doi:10.1016/S1470-2045(11)70149-8 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
