Glial Fibrillary Acidic Protein Astrocytopathy: Review of Pathogenesis, Imaging Features, and Radiographic Mimics
- PMID: 38844367
- PMCID: PMC11448981
- DOI: 10.3174/ajnr.A8236
Glial Fibrillary Acidic Protein Astrocytopathy: Review of Pathogenesis, Imaging Features, and Radiographic Mimics
Abstract
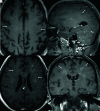
Glial fibrillary acidic protein (GFAP) astrocytopathy is a recently described autoimmune inflammatory disorder of the CNS characterized by the presence of specific antibodies targeting the intracellular filament protein in mature astrocytes. The pathogenesis is heterogeneous and poorly understood, with around 20%-34% of cases occurring as a paraneoplastic syndrome, most frequently associated with ovarian teratomas. It presents clinically as acute or subacute encephalomyelitis, and the diagnosis relies on imaging and detection of GFAP-Immunoglobulin (GFAP-IgG) in the CSF. Characteristic imaging findings include linear perivascular enhancement in the white matter extending in a radial pattern. Other imaging findings include periependymal enhancement, longitudinally extensive cord signal changes, intramedullary enhancement, optic neuritis, and papillitis. There is significant imaging overlap with other neuroinflammatory diseases like neuromyelitis optica spectrum disorder and lymphoproliferative conditions. GFAP astrocytopathy is characteristically responsive to steroids with, however, a significant rate of relapse. Currently, literature on this novel entity is limited with no established diagnostic criteria or standard treatment regimen. This comprehensive review explores the clinical, radiographic, and histopathologic aspects of GFAP astrocytopathy, shedding light on its complex nature and potential diagnostic challenges. The paper highlights the neuroimaging findings with a focus on differentiating GFAP astrocytopathy from other neuroinflammatory disorders.
© 2024 by American Journal of Neuroradiology.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
