New data-driven method to predict the therapeutic indication of redeemed prescriptions in secondary data sources: a case study on antiseizure medications users aged ≥65 identified in Danish registries
- PMID: 38844392
- PMCID: PMC11163620
- DOI: 10.1136/bmjopen-2023-080126
New data-driven method to predict the therapeutic indication of redeemed prescriptions in secondary data sources: a case study on antiseizure medications users aged ≥65 identified in Danish registries
Abstract
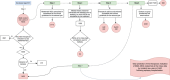
Objectives: We aimed to develop a new data-driven method to predict the therapeutic indication of redeemed prescriptions in secondary data sources using antiepileptic drugs among individuals aged ≥65 identified in Danish registries.
Design: This was an incident new-user register-based cohort study using Danish registers.
Setting: The study setting was Denmark and the study period was 2005-2017.
Participants: Participants included antiepileptic drug users in Denmark aged ≥65 with a confirmed diagnosis of epilepsy.
Primary and secondary outcome measures: Sensitivity served as the performance measure of the algorithm.
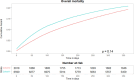
Results: The study population comprised 8609 incident new users of antiepileptic drugs. The sensitivity of the algorithm in correctly predicting the therapeutic indication of antiepileptic drugs in the study population was 65.3% (95% CI 64.4 to 66.2).
Conclusions: The algorithm demonstrated promising properties in terms of overall sensitivity for predicting the therapeutic indication of redeemed antiepileptic drugs by older individuals with epilepsy, correctly identifying the therapeutic indication for 6 out of 10 individuals using antiepileptic drugs for epilepsy.
Keywords: aged; epidemiology; epilepsy.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


Similar articles
-
An algorithm for drug-resistant epilepsy in Danish national registers.Brain. 2025 Mar 6;148(3):753-763. doi: 10.1093/brain/awae286. Brain. 2025. PMID: 39255058
-
Prenatal exposure to antiepileptic drugs and use of primary healthcare during childhood: a population-based cohort study in Denmark.BMJ Open. 2017 Jan 5;7(1):e012836. doi: 10.1136/bmjopen-2016-012836. BMJ Open. 2017. PMID: 28069620 Free PMC article.
-
Use of antiepileptic drugs during pregnancy and risk of spontaneous abortion and stillbirth: population based cohort study.BMJ. 2014 Aug 21;349:g5159. doi: 10.1136/bmj.g5159. BMJ. 2014. PMID: 25150301 Free PMC article.
-
Correlation of drug dose estimated from national prescription registers with mean blood level of antiseizure medication in pregnancy.Pharmacoepidemiol Drug Saf. 2024 Jun;33(6):e5811. doi: 10.1002/pds.5811. Pharmacoepidemiol Drug Saf. 2024. PMID: 38783423
-
Strategies for improving adherence to antiepileptic drug treatment in people with epilepsy.Cochrane Database Syst Rev. 2020 Oct 22;10(10):CD008312. doi: 10.1002/14651858.CD008312.pub4. Cochrane Database Syst Rev. 2020. PMID: 33089492 Free PMC article.
References
-
- Soeorg H, Sverrisdóttir E, Andersen M, et al. . The PHARMACOM-EPI framework for integrating Pharmacometric Modelling into Pharmacoepidemiological research using real-world data: application to assess death associated with valproate. Clin Pharmacol Ther 2022;111:840–56. 10.1002/cpt.2502 - DOI - PubMed
