BCAAs acutely drive glucose dysregulation and insulin resistance: role of AgRP neurons
- PMID: 38844453
- PMCID: PMC11156648
- DOI: 10.1038/s41387-024-00298-y
BCAAs acutely drive glucose dysregulation and insulin resistance: role of AgRP neurons
Abstract
Background: High-protein diets are often enriched with branched-chain amino acids (BCAAs) known to enhance protein synthesis and provide numerous physiological benefits, but recent studies reveal their association with obesity and diabetes. In support of this, protein or BCAA supplementation is shown to disrupt glucose metabolism while restriction improves it. However, it is not clear if these are primary, direct effects of BCAAs or secondary to other physiological changes during chronic manipulation of dietary BCAAs.
Methods: Three-month-old C57Bl/6 mice were acutely treated with either vehicle/BCAAs or BT2, a BCAA-lowering compound, and detailed in vivo metabolic phenotyping, including frequent sampling and pancreatic clamps, were conducted.
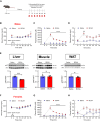
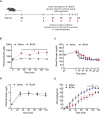
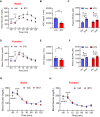
Results: Using a catheter-guided frequent sampling method in mice, here we show that a single infusion of BCAAs was sufficient to acutely elevate blood glucose and plasma insulin. While pre-treatment with BCAAs did not affect glucose tolerance, a constant infusion of BCAAs during hyperinsulinemic-euglycemic clamps impaired whole-body insulin sensitivity. Similarly, a single injection of BT2 was sufficient to prevent BCAA rise during fasting and markedly improve glucose tolerance in high-fat-fed mice, suggesting that abnormal glycemic control in obesity may be causally linked to high circulating BCAAs. We further show that chemogenetic over-activation of AgRP neurons in the hypothalamus, as present in obesity, significantly impairs glucose tolerance that is completely normalized by acute BCAA reduction. Interestingly, most of these effects were demonstrated only in male, but not in female mice.
Conclusion: These findings suggest that BCAAs per se can acutely impair glucose homeostasis and insulin sensitivity, thus offering an explanation for how they may disrupt glucose metabolism in the long-term as observed in obesity and diabetes. Our findings also reveal that AgRP neuronal regulation of blood glucose is mediated through BCAAs, further elucidating a novel mechanism by which brain controls glucose homeostasis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Torres-Leal FL, Fonseca-Alaniz MH, Teodoro GF, de Capitani MD, Vianna D, Pantaleao LC, et al. Leucine supplementation improves adiponectin and total cholesterol concentrations despite the lack of changes in adiposity or glucose homeostasis in rats previously exposed to a high-fat diet. Nutr Metab. 2011;8:62. doi: 10.1186/1743-7075-8-62. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

