PNPLA3 is a triglyceride lipase that mobilizes polyunsaturated fatty acids to facilitate hepatic secretion of large-sized very low-density lipoprotein
- PMID: 38844467
- PMCID: PMC11156938
- DOI: 10.1038/s41467-024-49224-x
PNPLA3 is a triglyceride lipase that mobilizes polyunsaturated fatty acids to facilitate hepatic secretion of large-sized very low-density lipoprotein
Abstract
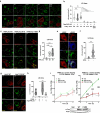
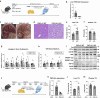
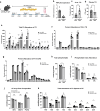
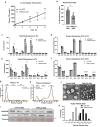
The I148M variant of PNPLA3 is closely associated with hepatic steatosis. Recent evidence indicates that the I148M mutant functions as an inhibitor of PNPLA2/ATGL-mediated lipolysis, leaving the role of wild-type PNPLA3 undefined. Despite showing a triglyceride hydrolase activity in vitro, PNPLA3 has yet to be established as a lipase in vivo. Here, we show that PNPLA3 preferentially hydrolyzes polyunsaturated triglycerides, mobilizing polyunsaturated fatty acids for phospholipid desaturation and enhancing hepatic secretion of triglyceride-rich lipoproteins. Under lipogenic conditions, mice with liver-specific knockout or acute knockdown of PNPLA3 exhibit aggravated liver steatosis and reduced plasma VLDL-triglyceride levels. Similarly, I148M-knockin mice show decreased hepatic triglyceride secretion during lipogenic stimulation. Our results highlight a specific context whereby the wild-type PNPLA3 facilitates the balance between hepatic triglyceride storage and secretion, and suggest the potential contribution of a loss-of-function by the I148M variant to the development of fatty liver disease in humans.
© 2024. The Author(s).
Conflict of interest statement
KM-B and DL are employed by and have shares in AstraZeneca. The remaining authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- DK124190/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- DK089178/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- R01 DK089178/DK/NIDDK NIH HHS/United States
- R56 DK089178/DK/NIDDK NIH HHS/United States
- DK109096/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

