Donor human milk versus infant formula for low-risk infants: a systematic review
- PMID: 38844541
- PMCID: PMC11798828
- DOI: 10.1038/s41390-024-03309-x
Donor human milk versus infant formula for low-risk infants: a systematic review
Abstract
Background: There is an increasing acceptance and use of donor human milk (DHM) in healthy infants. This review investigates the benefits and risks of mothers' own milk (MOM) supplementation with DHM compared to infant formula (IF) in moderate-late preterm (MLP) and early term (ET) infants.
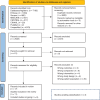
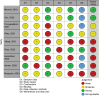
Methods: MEDLINE, EMBASE, CINAHL, Scopus, Cochrane CENTRAL and clinical trial registries were searched for studies published up to September 2023. The primary outcome was rates of exclusive breastfeeding (EBF). Certainty of evidence was assessed using GRADE framework. RoB1 and EPHPP were used to assess risk of bias for controlled trials and observational studies, respectively.
Results: Eleven studies involving total of 10,147 infants and six ongoing trials were identified. Studies were of low quality, and the certainty of evidence was assessed as very low. Three studies suggested benefits of DHM compared to IF on EBF at discharge, while two suggested no difference. No clear effect was observed on EBF duration, any breastfeeding, hypoglycemia and morbidity. No health risks were reported.
Conclusion: The effect of supplementing MOM with DHM instead of IF on EBF and other health outcomes is unclear. High-quality studies are required to determine the potential benefits or risks of DHM supplementation in this population.
Impact: We identified 11 relevant studies reporting on supplementation of mothers' own milk (MOM) with donor human milk (DHM) compared to infant formula (IF). Studies were of low quality, had heterogeneous outcome definitions and were geographically limited; all except two were observational studies. Limited evidence showed no clear difference on rates of exclusive breastfeeding and other health outcomes. No potential risks were reported. The increasing acceptance and use of DHM in healthy infants highlights the need for future high-quality studies.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Catherine Fiddes and Nicholas Embleton are investigators in one of the ongoing studies identified in this review. No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this review.
Figures

References
-
- Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet379, 2162–2172 (2012). - PubMed
-
- Mitha, A. et al. Neonatal morbidities in infants born late preterm at 35-36 weeks of gestation: a Swedish nationwide population-based study. J. Pediatr.233, 43–50 (2021). - PubMed
-
- Delnord, M. & Zeitlin, J. Epidemiology of late preterm and early term births – An international perspective. Semin. Fetal. Neonatal. Med.24, 3–10 (2019). - PubMed
-
- Helle, E. et al. Morbidity and health care costs after early term birth. Paediatr. Perinat. Epidemiol.30, 533–540 (2016). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

