Looping forward: exploring R-loop processing and therapeutic potential
- PMID: 38844597
- PMCID: PMC11771710
- DOI: 10.1002/1873-3468.14947
Looping forward: exploring R-loop processing and therapeutic potential
Abstract
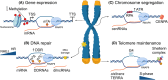
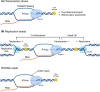
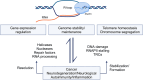
Recently, there has been increasing interest in the complex relationship between transcription and genome stability, with specific attention directed toward the physiological significance of molecular structures known as R-loops. These structures arise when an RNA strand invades into the DNA duplex, and their formation is involved in a wide range of regulatory functions affecting gene expression, DNA repair processes or cell homeostasis. The persistent presence of R-loops, if not effectively removed, contributes to genome instability, underscoring the significance of the factors responsible for their resolution and modification. In this review, we provide a comprehensive overview of how R-loop processing can drive either a beneficial or a harmful outcome. Additionally, we explore the potential for manipulating such structures to devise rationalized therapeutic strategies targeting the aberrant accumulation of R-loops.
Keywords: DNA damage; DNA repair; R‐loops; genome instability; transcription.
© 2024 The Author(s). FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

