The effects of sodium hydrogen carbonate ingestion during the recovery period between two 200-m front-crawl time trials
- PMID: 38844672
- PMCID: PMC11519313
- DOI: 10.1007/s00421-024-05522-2
The effects of sodium hydrogen carbonate ingestion during the recovery period between two 200-m front-crawl time trials
Abstract
Purpose: The aim of this study was to determine how sodium hydrogen carbonate (NaHCO3) ingestion during a 1-h recovery period after a 200-m front-crawl swim affects blood-gas levels, acid-base balance, and performance during a successive trial.
Methods: Fourteen national-level male swimmers (age: 21 ± 3 years, body mass (BM):77 ± 10 kg, stature: 181 ± 7 cm) performed four maximal 200-m front-crawl tests. On one of the two days, the swimmers swam two 200-m tests with a 1-h recovery break, during which they drank water (WATER); on the other day, they performed the same protocol but consumed 0.3 g min-1 NaHCO3 solution during the recovery break (NaHCO3).
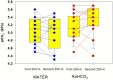
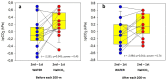
Results: The ingestion of NaHCO3 before the second test had no effect on swim time despite a greater [ ] (19.2 ± 2.3 mmol L-1) than that measured during the first test (NaHCO3) (14.5 ± 1.1 mmol L-1) and the other two tests (WATER) (12.7 ± 2.4 and 14.8 ± 1.5 mmol L-1; F = 18.554; p = 0.000) and a higher blood pH (7.46 ± 0.03) than that measured during the first test (NaHCO3) (7.39 ± 0.02) and the other two tests (WATER) (7.16 ± 0.04 and 7.20 ± 0.05); (F = 5.255; p = 0.004). An increase in blood pCO2 (0.2 ± 0.3 kPa) between both tests (NaHCO3) compared to unchanged pCO2 values (- 0.1 ± 0.3 kPa) between the other two tests (WATER) (t = - 2.984; p = 0.011; power = 0.741) was confirmed.
Conclusions: NaHCO3 ingestion during the recovery period between two 200-m front-crawl time trials had a strong buffering effect that did not positively affect performance. An increase in pCO2 may have counterbalanced this impact.
Keywords: Acidosis; Acid–base balance; Blood alkalosis; Blood gases.
© 2024. The Author(s).
Conflict of interest statement
There was no conflict of interest, financial or otherwise, are declared by authors.
Figures
Similar articles
-
Sodium bicarbonate ingestion and repeated swim sprint performance.J Strength Cond Res. 2010 Nov;24(11):3105-11. doi: 10.1519/JSC.0b013e3181f55eb1. J Strength Cond Res. 2010. PMID: 20881504 Clinical Trial.
-
Effects of post-exercise sodium bicarbonate ingestion on acid-base balance recovery and time-to-exhaustion running performance: a randomised crossover trial in recreational athletes.Appl Physiol Nutr Metab. 2021 Sep;46(9):1111-1118. doi: 10.1139/apnm-2020-1120. Epub 2021 Mar 17. Appl Physiol Nutr Metab. 2021. PMID: 33730517 Clinical Trial.
-
Neither an Individualised Nor a Standardised Sodium Bicarbonate Strategy Improved Performance in High-Intensity Repeated Swimming, or a Subsequent 200 m Swimming Time Trial in Highly Trained Female Swimmers.Nutrients. 2024 Sep 16;16(18):3123. doi: 10.3390/nu16183123. Nutrients. 2024. PMID: 39339723 Free PMC article. Clinical Trial.
-
Effects of acute alkalosis and acidosis on performance: a meta-analysis.Sports Med. 2011 Oct 1;41(10):801-14. doi: 10.2165/11591440-000000000-00000. Sports Med. 2011. PMID: 21923200 Review.
-
Acute and chronic effect of sodium bicarbonate ingestion on Wingate test performance: a systematic review and meta-analysis.J Sports Sci. 2019 Apr;37(7):762-771. doi: 10.1080/02640414.2018.1524739. Epub 2018 Oct 13. J Sports Sci. 2019. PMID: 30319077
References
-
- Åstrand P-O, Rodahl K, Dahl HA, Strømme SB (2003) Textbook of work physiology. Physiological bases of exercise, Human Kinetics, Champaign, IL
-
- Bishop D, Claudius B (2005) Effects of induced metabolic alkalosis on prolonged intermittent-sprint performance. Med Sci Sports Exerc 37(5):759–767. 10.1249/01.MSS.0000161803.44656.3C - PubMed
-
- Böning D, Klarholz C, Himmelsbach B, Hütler M, Maassen N (2007) Extracellular bicarbonate and non-bicarbonate buffering against lactic acid during and after exercise. Eur J Appl Physiol 100(4):457–467. 10.1007/s00421-007-0453-4 - PubMed
-
- Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381. 10.1249/00005768-198205000-00012 - PubMed
-
- Burke LM (2013) Practical considerations for bicarbonate loading and sports performance. Nestle Nutr Inst Workshop Ser 75:15–26. 10.1159/000345814 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

