Trans-cyclooctene-a Swiss army knife for bioorthogonal chemistry: exploring the synthesis, reactivity, and applications in biomedical breakthroughs
- PMID: 38844698
- PMCID: PMC11156836
- DOI: 10.1186/s41181-024-00275-x
Trans-cyclooctene-a Swiss army knife for bioorthogonal chemistry: exploring the synthesis, reactivity, and applications in biomedical breakthroughs
Abstract
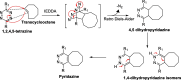
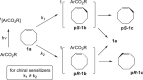
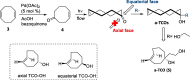
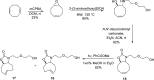
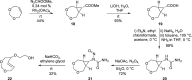
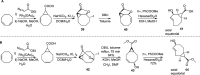
Background: Trans-cyclooctenes (TCOs) are highly strained alkenes with remarkable reactivity towards tetrazines (Tzs) in inverse electron-demand Diels-Alder reactions. Since their discovery as bioorthogonal reaction partners, novel TCO derivatives have been developed to improve their reactivity, stability, and hydrophilicity, thus expanding their utility in diverse applications.
Main body: TCOs have garnered significant interest for their applications in biomedical settings. In chemical biology, TCOs serve as tools for bioconjugation, enabling the precise labeling and manipulation of biomolecules. Moreover, their role in nuclear medicine is substantial, with TCOs employed in the radiolabeling of peptides and other biomolecules. This has led to their utilization in pretargeted nuclear imaging and therapy, where they function as both bioorthogonal tags and radiotracers, facilitating targeted disease diagnosis and treatment. Beyond these applications, TCOs have been used in targeted cancer therapy through a "click-to-release" approach, in which they act as key components to selectively deliver therapeutic agents to cancer cells, thereby enhancing treatment efficacy while minimizing off-target effects. However, the search for a suitable TCO scaffold with an appropriate balance between stability and reactivity remains a challenge.
Conclusions: This review paper provides a comprehensive overview of the current state of knowledge regarding the synthesis of TCOs, and its challenges, and their development throughout the years. We describe their wide ranging applications as radiolabeled prosthetic groups for radiolabeling, as bioorthogonal tags for pretargeted imaging and therapy, and targeted drug delivery, with the aim of showcasing the versatility and potential of TCOs as valuable tools in advancing biomedical research and applications.
Keywords: Trans-cyclooctene; Bioorthogonal chemistry; In vivo ligation; Pretargeted imaging and therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






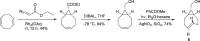

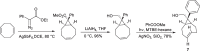
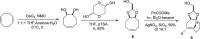









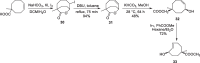
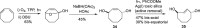





References
-
- Allinger NL, Sprague JT. Conformational analysis. LXXXIV. Study of the structures and energies of some alkenes and cycloalkenes by the force field method. J Am Chem Soc. 2002;94(16):5734–5747. doi: 10.1021/ja00771a034. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
