Amniotic fluid stem cell-derived extracellular vesicles educate type 2 conventional dendritic cells to rescue autoimmune disorders in a multiple sclerosis mouse model
- PMID: 38844736
- PMCID: PMC11156524
- DOI: 10.1002/jev2.12446
Amniotic fluid stem cell-derived extracellular vesicles educate type 2 conventional dendritic cells to rescue autoimmune disorders in a multiple sclerosis mouse model
Abstract
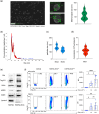
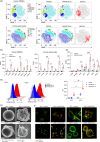
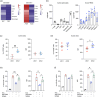
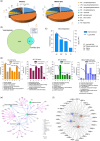
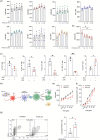
Dendritic cells (DCs) are essential orchestrators of immune responses and represent potential targets for immunomodulation in autoimmune diseases. Human amniotic fluid secretome is abundant in immunoregulatory factors, with extracellular vesicles (EVs) being a significant component. However, the impact of these EVs on dendritic cells subsets remain unexplored. In this study, we investigated the interaction between highly purified dendritic cell subsets and EVs derived from amniotic fluid stem cell lines (HAFSC-EVs). Our results suggest that HAFSC-EVs are preferentially taken up by conventional dendritic cell type 2 (cDC2) through CD29 receptor-mediated internalization, resulting in a tolerogenic DC phenotype characterized by reduced expression and production of pro-inflammatory mediators. Furthermore, treatment of cDC2 cells with HAFSC-EVs in coculture systems resulted in a higher proportion of T cells expressing the regulatory T cell marker Foxp3 compared to vehicle-treated control cells. Moreover, transfer of HAFSC-EV-treated cDC2s into an EAE mouse model resulted in the suppression of autoimmune responses and clinical improvement. These results suggest that HAFSC-EVs may serve as a promising tool for reprogramming inflammatory cDC2s towards a tolerogenic phenotype and for controlling autoimmune responses in the central nervous system, representing a potential platform for the study of the effects of EVs in DC subsets.
Keywords: amniotic fluid stem cells; autoimmune diseases; conventional dendritic cell type 2 (cDC2); dendritic cells; experimental autoimmune encephalomyelitis (EAE); extracellular vesicles; tolerogenic phenotype.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Awasthi, A. , Carrier, Y. , Peron, J. P. , Bettelli, E. , Kamanaka, M. , Flavell, R. A. , Kuchroo, V. K. , Oukka, M. , & Weiner, H. L. (2007). A dominant function for interleukin 27 in generating interleukin 10‐producing anti‐inflammatory T cells. Nature Immunology, 8, 1380–1389. - PubMed
-
- Balbi, C. , Piccoli, M. , Barile, L. , Papait, A. , Armirotti, A. , Principi, E. , Reverberi, D. , Pascucci, L. , Becherini, P. , Varesio, L. , Mogni, M. , Coviello, D. , Bandiera, T. , Pozzobon, M. , Cancedda, R. , & Bollini, S. (2017). First characterization of human amniotic fluid stem cell extracellular vesicles as a powerful paracrine tool endowed with regenerative potential. Stem Cells Translational Medicine, 6, 1340–1355. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

