KSHV infection of B cells primes protective T cell responses in humanized mice
- PMID: 38844783
- PMCID: PMC11156630
- DOI: 10.1038/s41467-024-49209-w
KSHV infection of B cells primes protective T cell responses in humanized mice
Abstract
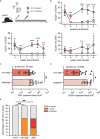
Kaposi sarcoma associated herpesvirus (KSHV) is associated with around 1% of all human tumors, including the B cell malignancy primary effusion lymphoma (PEL), in which co-infection with the Epstein Barr virus (EBV) can almost always be found in malignant cells. Here, we demonstrate that KSHV/EBV co-infection of mice with reconstituted human immune systems (humanized mice) leads to IgM responses against both latent and lytic KSHV antigens, and expansion of central and effector memory CD4+ and CD8+ T cells. Among these, KSHV/EBV dual-infection allows for the priming of CD8+ T cells that are specific for the lytic KSHV antigen K6 and able to kill KSHV/EBV infected B cells. This suggests that K6 may represent a vaccine antigen for the control of KSHV and its associated pathologies in high seroprevalence regions, such as Sub-Saharan Africa.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

