Precision-guided treatment in high-risk pediatric cancers
- PMID: 38844796
- PMCID: PMC11271405
- DOI: 10.1038/s41591-024-03044-0
Precision-guided treatment in high-risk pediatric cancers
Abstract
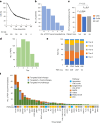
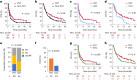
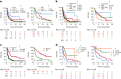
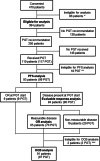
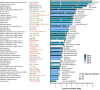
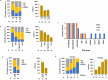
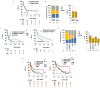
Recent research showed that precision medicine can identify new treatment strategies for patients with childhood cancers. However, it is unclear which patients will benefit most from precision-guided treatment (PGT). Here we report consecutive data from 384 patients with high-risk pediatric cancer (with an expected cure rate of less than 30%) who had at least 18 months of follow-up on the ZERO Childhood Cancer Precision Medicine Program PRecISion Medicine for Children with Cancer (PRISM) trial. A total of 256 (67%) patients received PGT recommendations and 110 (29%) received a recommended treatment. PGT resulted in a 36% objective response rate and improved 2-year progression-free survival compared with standard of care (26% versus 12%; P = 0.049) or targeted agents not guided by molecular findings (26% versus 5.2%; P = 0.003). PGT based on tier 1 evidence, PGT targeting fusions or commenced before disease progression had the greatest clinical benefit. Our data show that PGT informed by comprehensive molecular profiling significantly improves outcomes for children with high-risk cancers. ClinicalTrials.gov registration: NCT03336931.
© 2024. The Author(s).
Conflict of interest statement
All authors, except P.G.E., S.-L.K., L.M.S.L., J.R.H., N.G.G. and D.S.Z., declare no competing interests. L.M.S.L. received consulting and advisory board fees from Bayer; J.R.H. received fees from Bayer, AstraZeneca/Alexion and Boxer Capital; N.G.G received fees to his institution from Bayer and Day One; D.S.Z. received fees from Bayer, AstraZeneca/Alexion, Accendatech, Novartis, Day One, FivePhusion, Amgen and Norgine, and research support from Accendatech. P.G.E. and S.-L.K. are recipients of a share in milestone and royalty payments for venetoclax development.
Figures




References
-
- van Tilburg, C. M. et al. The pediatric precision oncology INFORM registry: clinical outcome and benefit for patients with very high-evidence targets. Cancer Discov.11, 2764–2779 (2021). 10.1158/2159-8290.CD-21-0094 - DOI - PMC - PubMed
-
- Berlanga, P. et al. The European MAPPYACTS trial: precision medicine program in pediatric and adolescent patients with recurrent malignancies. Cancer Discov.12, 1266–1281 (2022). 10.1158/2159-8290.CD-21-1136 - DOI - PMC - PubMed
MeSH terms
Associated data
Grants and funding
- APP2019056/Department of Health | National Health and Medical Research Council (NHMRC)
- APP2017898/Department of Health | National Health and Medical Research Council (NHMRC)
- APP2018642/Department of Health | National Health and Medical Research Council (NHMRC)
- APP1157871/Department of Health | National Health and Medical Research Council (NHMRC)
- 2021/TPG2112/Cancer Institute NSW (Cancer Institute New South Wales)
LinkOut - more resources
Full Text Sources
Medical

