Vedolizumab for the prevention of intestinal acute GVHD after allogeneic hematopoietic stem cell transplantation: a randomized phase 3 trial
- PMID: 38844797
- PMCID: PMC11333288
- DOI: 10.1038/s41591-024-03016-4
Vedolizumab for the prevention of intestinal acute GVHD after allogeneic hematopoietic stem cell transplantation: a randomized phase 3 trial
Abstract
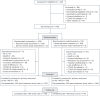
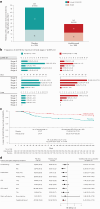
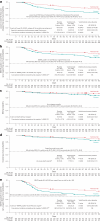
Acute graft-versus-host disease (aGVHD) of the lower gastrointestinal (GI) tract is a major cause of morbidity and mortality in patients receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT). Vedolizumab is a gut-selective anti-α4β7 integrin monoclonal antibody that reduces gut inflammation by inhibiting migration of GI-homing T lymphocytes. The efficacy and safety of vedolizumab added to standard GVHD prophylaxis (calcineurin inhibitor plus methotrexate/mycophenolate mofetil) was evaluated for prevention of lower-GI aGVHD after unrelated donor allo-HSCT in a randomized, double-blind, placebo-controlled phase 3 trial. Enrollment closed early during the COVID-19 pandemic with 343 patients randomized (n = 174 vedolizumab, n = 169 placebo), and 333 received ≥1 intravenous dose of 300 mg vedolizumab (n = 168) or placebo (n = 165) and underwent allo-HSCT. The primary end point was met; Kaplan-Meier (95% confidence interval) estimated rates of lower-GI aGVHD-free survival by day +180 after allo-HSCT were 85.5% (79.2-90.1) with vedolizumab versus 70.9% (63.2-77.2) with placebo (hazard ratio, 0.45; 95% confidence interval, 0.27-0.73; P < 0.001). For the 5 key secondary efficacy end points analyzed by day +180 after allo-HSCT, rates of lower-GI aGVHD-free and relapse-free survival and grade C-D aGVHD-free survival were significantly higher with vedolizumab versus placebo. No significant treatment differences were found for the other key secondary end points of non-relapse mortality, overall survival and grade B-D aGVHD-free survival, respectively. Incidence of treatment-related serious adverse events analyzed in patients receiving ≥1 dose of study treatment (n = 334) was 6.5% (n = 11 of 169) vedolizumab versus 8.5% (n = 14 of 165) placebo. When added to standard calcineurin inhibitor-based GVHD prevention, lower-GI aGVHD-free survival was significantly higher with vedolizumab versus placebo. ClinicalTrials.gov identifier: NCT03657160 .
© 2024. Takeda Development Center Americas, Inc.
Conflict of interest statement
Y.-B.C. has received consulting fees from Incyte, Novartis, Magenta, Daiichi, Celularity, Equillium, Vor Biopharma and Pharmacosmos. M.M. has received research funding from Sanofi and Jazz Pharmaceuticals, and honoraria from Sanofi, Jazz, Celgene, Bristol Myers Squibb, Takeda, Amgen, Astellas, Novartis, Adaptive Biotechnologies, Oncopeptides, Pfizer, GSK and Gilead Sciences. R.Z. has received consulting fees from Incyte, Novartis, MNK, Sanofi and VectivBio. T.T. has received research funding from Kyowa Kirin, Sanofi, Asahi Kasei Pharma, Chugai, Astellas, Eisai, Fuji Pharma, Nippon Shinyaku, Luca Science, Sumitomo Pharma, ONO, Shionogi, Priothera and Otsuka, and honoraria from Kyowa Kirin, Merck Sharp & Dohme, Bristol Myers Squibb, AbbVie and Celgene; participated in advisory boards for Merck Sharp & Dohme, Takeda and Novartis; and received non-financial support from Janssen, Novartis, Meiji Seika Pharma, Daiichi Sankyo, AstraZeneca and Roche Diagnostics. O.J. has participated in an advisory board for Ascentage. J.M. has participated in advisory boards for F2G, Mundipharma, Takeda, Pfizer, Gilead Sciences, Basilea and Astellas, and received speaker honoraria from F2G, Mundipharma, Takeda, Pfizer, Gilead Sciences and Basilea. D.P. has participated in advisory boards for Novartis, BMS Celgene, Jazz and Gilead, and received consulting fees from Bastion Educational. Y.F. has participated in advisory boards for Celgene, Takeda, Novartis, Otsuka and Pfizer, and received speaker honoraria from Celgene, Takeda, Novartis and Pfizer; travel grants from Celgene, Takeda, Novartis and AbbVie; and consulting fees from Dava Oncology, Arog and AbbVie. J.C., H.C., G.R. and J.J. are employees of Takeda and hold Takeda stock/stock options.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

