Microbial metabolite sodium butyrate enhances the anti-tumor efficacy of 5-fluorouracil against colorectal cancer by modulating PINK1/Parkin signaling and intestinal flora
- PMID: 38844824
- PMCID: PMC11156851
- DOI: 10.1038/s41598-024-63993-x
Microbial metabolite sodium butyrate enhances the anti-tumor efficacy of 5-fluorouracil against colorectal cancer by modulating PINK1/Parkin signaling and intestinal flora
Abstract
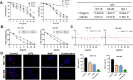
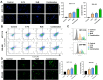
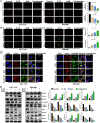
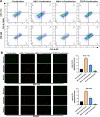
Colorectal cancer (CRC) is a prevalent global health issue, with 5-fluorouracil (5-FU) being a commonly used chemotherapeutic agent for its treatment. However, the efficacy of 5-FU is often hindered by drug tolerance. Sodium butyrate (NaB), a derivative of intestinal flora, has demonstrated anti-cancer properties both in vitro and in vivo through pro-apoptotic effects and has shown promise in improving outcomes when used in conjunction with traditional chemotherapy agents. This study seeks to evaluate the impact and potential mechanisms of NaB in combination with 5-FU on CRC. We employed a comprehensive set of assays, including CCK-8, EdU staining, Hoechst 33258 staining, flow cytometry, ROS assay, MMP assay, immunofluorescence, and mitophagy assay, to detect the effect of NaB on the biological function of CRC cells in vitro. Western blotting and immunohistochemistry were used to verify the above experimental results. The xenograft tumor model was established to evaluate the in vivo anti-CRC activity of NaB. Subsequently, 16S rRNA gene sequencing was used to analyze the intestinal flora. The findings of our study demonstrate that sodium butyrate (NaB) exerts inhibitory effects on tumor cell proliferation and promotes tumor cell apoptosis in vitro, while also impeding tumor progression in vivo through the enhancement of the mitophagy pathway. Furthermore, the combined treatment of NaB and 5-fluorouracil (5-FU) yielded superior therapeutic outcomes compared to monotherapy with either agent. Moreover, this combination therapy resulted in the specific enrichment of Bacteroides, LigiLactobacillus, butyric acid-producing bacteria, and acetic acid-producing bacteria in the intestinal microbiota. The improvement in the intestinal microbiota contributed to enhanced therapeutic outcomes and reduced the adverse effects of 5-FU. Taken together, these findings indicate that NaB, a histone acetylation inhibitor synthesized through intestinal flora fermentation, has the potential to significantly enhance the therapeutic efficacy of 5-FU in CRC treatment and improve the prognosis of CRC patients.
Keywords: Apoptosis; Colorectal cancer; PINK1/Parkin; Reactive oxygen species; Sodium butyrate.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hamouda N, Sano T, Oikawa Y, Ozaki T, Shimakawa M, Matsumoto K, Amagase K, Higuchi K, Kato S. Apoptosis, dysbiosis and expression of inflammatory cytokines are sequential events in the development of 5-fluorouracil-induced intestinal mucositis in mice. Basic Clin. Pharmacol. Toxicol. 2017;121:159–168. doi: 10.1111/bcpt.12793. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

