An improved method to study Phytophthora cinnamomi Rands zoospores interactions with host
- PMID: 38844843
- PMCID: PMC11154991
- DOI: 10.1186/s12870-024-05205-2
An improved method to study Phytophthora cinnamomi Rands zoospores interactions with host
Abstract
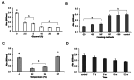
Phytophthora cinnamomi Rands is a highly prevalent phytopathogen worldwide, ranking among the top ten in terms of distribution. It inflicts crown rot, canker, and root rot on numerous plant species, significantly impacting the biodiversity of both flora and fauna within affected environments. With a host range spanning over 5,000 species, including important plants like Quercus suber, Quercus ilex, Castanea sativa, and commercially significant crops such as avocado (Persea americana), maize (Zea mays), and tomato (Solanum lycopersicum), Phytophthora cinnamomi poses a substantial threat to agriculture and ecosystems. The efficient dissemination of the oomycete relies on its short-lived asexually motile zoospores, which depend on water currents to infect host roots. However, managing these zoospores in the laboratory has long been challenging due to the complexity of the life cycle. Current protocols involve intricate procedures, including alternating cycles of growth, drought, and flooding. Unfortunately, these artificial conditions often result in a rapid decline in virulence, necessitating additional steps to maintain infectivity during cultivation. In our research, we sought to address this challenge by investigating zoospore survival under various conditions. Our goal was to develop a stable stock of zoospores that is both easily deployable and highly infective. Through direct freezing in liquid nitrogen, we have successfully preserved their virulence. This breakthrough eliminates the need for repeated culture transfers, simplifying the process of plant inoculation. Moreover, it enables more comprehensive studies of Phytophthora cinnamomi and its interactions with host plants.
Keywords: Phytophthora cinnamomi Rands; Quercus Sp; Solanum lycopersicum; Zoospores; qRT-PCR.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Shakya SK, Grünwald NJ, Fieland VJ, et al. Phylogeography of the wide-host range panglobal plant pathogen Phytophthora cinnamomi. Mol Ecol. 2021;30(20). 10.1111/mec.16109 - PubMed
-
- Corcobado T, Cubera E, Pérez-Sierra A, Jung T, Solla A. First report of Phytophthora gonapodyides involved in the decline of Quercus ilex in xeric conditions in Spain. New Dis Rep. 2010;22(1). 10.5197/j.2044-0588.2010.022.033
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

