Safety and adherence of bictegravir/emtricitabine/tenofovir alafenamide for HIV post-exposure prophylaxis among adults in Guiyang China: a prospective cohort study
- PMID: 38844855
- PMCID: PMC11157740
- DOI: 10.1186/s12879-024-09407-9
Safety and adherence of bictegravir/emtricitabine/tenofovir alafenamide for HIV post-exposure prophylaxis among adults in Guiyang China: a prospective cohort study
Abstract
Background: The effectiveness of post-exposure prophylaxis (PEP) depends on participants adherence, making it crucial to assess and compare regimen options to enhance human immunodeficiency virus (HIV) prophylaxis strategies. However, no prospective study in China has shown that the completion rate and adherence of single-tablet regimens in HIV PEP are higher than those of multi-tablet preparations. Therefore, this study aimed to assess the completion rate and adherence of two HIV PEP regimens.
Methods: In this single-center, prospective, open-label cohort study, we included 179 participants from May 2022 to March 2023 and analyzed the differences in the 28-day medication completion rate, adherence, safety, tolerance, and effectiveness of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) and tenofovir disoproxil fumarate, emtricitabine, and dolutegravir (TDF/FTC + DTG).
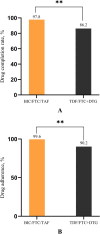
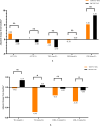
Results: The PEP completion rate and adherence were higher in the BIC/FTC/TAF group than in the TDF/FTC + DTG group (completion rate: 97.8% vs. 82.6%, P = 0.009; adherence: 99.6 ± 2.82% vs. 90.2 ± 25.29%, P = 0.003). The incidence of adverse reactions in the BIC/FTC/TAF and TDF/FTC + DTG groups was 15.2% and 10.3% (P = 0.33), respectively. In the TDF/FTC + DTG group, one participant stopped PEP owing to adverse reactions (1.1%). No other participants stopped PEP due to adverse events.
Conclusions: BIC/FTC/TAF and TDF/FTC + DTG have good safety and tolerance as PEP regimens. BIC/FTC/TAF has a higher completion rate and increased adherence, thus, is recommended as a PEP regimen. These findings emphasize the importance of regimen choice in optimizing PEP outcomes.
Trial registration: The study was registered in the Chinese Clinical Trial Registry (registration number: ChiCTR2200059994(2022-05-14), https://www.chictr.org.cn/bin/project/edit?pid=167391 ).
Keywords: Bictegravir/emtricitabine/tenofovir alafenamide; HIV post-exposure prophylaxis; Integrase strand transfer inhibitors; Safety; Single-tablet regimen.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Clinical Pharmacology of the Single Tablet Regimen Bictegravir/Emtricitabine/Tenofovir Alafenamide in the evolving era of antiretroviral therapies.New Microbiol. 2024 Nov;47(3):243-250. New Microbiol. 2024. PMID: 39560035 Review.
-
Rapid Initiation of Antiretroviral Therapy With Coformulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide Versus Efavirenz, Lamivudine, and Tenofovir Disoproxil Fumarate in HIV-Positive Men Who Have Sex With Men in China: Week 48 Results of the Multicenter, Randomized Clinical Trial.Clin Infect Dis. 2024 Jul 19;79(1):169-176. doi: 10.1093/cid/ciae012. Clin Infect Dis. 2024. PMID: 38236137 Clinical Trial.
-
Comparing the long-term effectiveness and safety of dolutegravir/lamivudine versus bictegravir/emtricitabine/tenofovir alafenamide fumarate as first-line regimens: a real life multicentre cohort.J Antimicrob Chemother. 2025 Jan 3;80(1):175-177. doi: 10.1093/jac/dkae392. J Antimicrob Chemother. 2025. PMID: 39478330
-
Rapid initiation of bictegravir/emtricitabine/tenofovir alafenamide as first-line therapy in HIV infection. A prospective study.J Antimicrob Chemother. 2024 Sep 3;79(9):2343-2353. doi: 10.1093/jac/dkae235. J Antimicrob Chemother. 2024. PMID: 39045754 Clinical Trial.
-
Effectiveness, safety and discontinuation rates of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) in people with HIV using real-world data: a systematic review and meta-analysis.J Antimicrob Chemother. 2024 Aug 1;79(8):1775-1783. doi: 10.1093/jac/dkae138. J Antimicrob Chemother. 2024. PMID: 38758191
References
-
- UNAIDS. The path that ends AIDS [EB/OL]. 2023. https://ncaids.chinacdc.cn/xxgx/yqbh/202307/W020230714332118740538.pdf. Accessed 30 Jan 2024.
-
- National Bureau of Disease Control and Prevention. [EB/OL]. 2019. https://www.gov.cn/xinwen/2019-10/13/content_5439036.htm. Accessed 27 May 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

