Systemic antibiotics for Pseudomonas aeruginosa infection in outpatients with non-hospitalised exacerbations of pre-existing lung diseases: a randomised clinical trial
- PMID: 38844921
- PMCID: PMC11157704
- DOI: 10.1186/s12931-024-02860-9
Systemic antibiotics for Pseudomonas aeruginosa infection in outpatients with non-hospitalised exacerbations of pre-existing lung diseases: a randomised clinical trial
Abstract
Background: The effect of dual systemic antibiotic therapy against Pseudomonas aeruginosa in patients with pre-existing lung disease is unknown. To assess whether dual systemic antibiotics against P. aeruginosa in outpatients with COPD, non-cystic fibrosis (non-CF) bronchiectasis, or asthma can improve outcomes.
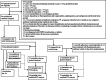
Methods: Multicenter, randomised, open-label trial conducted at seven respiratory outpatient clinics in Denmark. Outpatients with COPD, non-CF bronchiectasis, or asthma with a current P. aeruginosa-positive lower respiratory tract culture (clinical routine samples obtained based on symptoms of exacerbation not requiring hospitalisation), regardless of prior P. aeruginosa-status, no current need for hospitalisation, and at least two moderate or one hospitalisation-requiring exacerbation within the last year were eligible. Patients were assigned 1:1 to 14 days of dual systemic anti-pseudomonal antibiotics or no antibiotic treatment. Primary outcome was time to prednisolone or antibiotic-requiring exacerbation or death from day 20 to day 365.
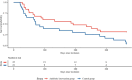
Results: The trial was stopped prematurely based in lack of recruitment during the COVID-19 pandemic, this decision was endorsed by the Data and Safety Monitoring Board. Forty-nine outpatients were included in the study. There was a reduction in risk of the primary outcome in the antibiotic group compared to the control group (HR 0.51 (95%CI 0.27-0.96), p = 0.037). The incidence of admissions with exacerbation within one year was 1.1 (95%CI 0.6-1.7) in the dual antibiotic group vs. 2.9 (95%CI 1.3-4.5) in the control group, p = 0.037.
Conclusions: Use of dual systemic antibiotics for 14 days against P. aeruginosa in outpatients with chronic lung diseases and no judged need for hospitalisation, improved clinical outcomes markedly. The main limitation was the premature closure of the trial.
Trial registration: ClinicalTrials.gov, NCT03262142, registration date 2017-08-25.
© 2024. The Author(s).
Conflict of interest statement
The first and last author have no conflicts of interest. CSU reports personal fees from AstraZeneca, GlaxoSmithKline, TEVA, Novartis, Boehringer Ingelheim, TFF Pharmaceuticals, Berlin Chemie, Orion Pharma, Sanofi, Takeda, Pfizer and Chiesi outside of the submitted work. All other authors declare no competing interests.
Figures
References
-
- Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A Comprehensive Analysis of the Impact of Pseudomonas aeruginosa Colonisation on Prognosis in Adult Bronchiectasis. Ann Am Thorac Soc. 2015;12:1602–1611. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical