Low clinical impact of HIV drug resistance mutations in oral pre-exposure prophylaxis: a systematic review and meta-analysis
- PMID: 38844950
- PMCID: PMC11155065
- DOI: 10.1186/s12981-024-00627-2
Low clinical impact of HIV drug resistance mutations in oral pre-exposure prophylaxis: a systematic review and meta-analysis
Abstract
Introduction: Despite the widespread use of pre-exposure prophylaxis (PrEP) in preventing human immunodeficiency virus (HIV) transmission, scant information on HIV drug resistance mutations (DRMs) has been gathered over the past decade. This review aimed to estimate the pooled prevalence of pre-exposure prophylaxis and its two-way impact on DRM.
Methods: We systematically reviewed studies on DRM in pre-exposure prophylaxis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 guidelines. PubMed, Cochrane, and SAGE databases were searched for English-language primary studies published between January 2001 and December 2023. The initial search was conducted on 9 August 2021 and was updated through 31 December 2023 to ensure the inclusion of the most recent findings. The registration number for this protocol review was CRD42022356061.
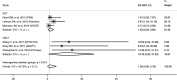
Results: A total of 26,367 participants and 562 seroconversion cases across 12 studies were included in this review. The pooled prevalence estimate for all mutations was 6.47% (95% Confidence Interval-CI 3.65-9.93), while Tenofovir Disoproxil Fumarate/Emtricitabine-associated drug resistance mutation prevalence was 1.52% (95% CI 0.23-3.60) in the pre-exposure prophylaxis arm after enrolment. A subgroup analysis, based on the study population, showed the prevalence in the heterosexual and men who have sex with men (MSM) groups was 5.53% (95% CI 2.55-9.40) and 7.47% (95% CI 3.80-12.11), respectively. Notably, there was no significant difference in the incidence of DRM between the pre-exposure prophylaxis and placebo groups (log-OR = 0.99, 95% CI -0.20 to 2.18, I2 = 0%; p = 0.10).
Discussion: Given the constrained prevalence of DRM, the World Health Organization (WHO) advocates the extensive adoption of pre-exposure prophylaxis. Our study demonstrated no increased risk of DRM with pre-exposure prophylaxis (p > 0.05), which is consistent with these settings. These findings align with the previous meta-analysis, which reported a 3.14-fold higher risk in the pre-exposure prophylaxis group than the placebo group, although the observed difference did not reach statistical significance (p = 0.21).
Conclusions: Despite the low prevalence of DRM, pre-exposure prophylaxis did not significantly increase the risk of DRM compared to placebo. However, long-term observation is required to determine further disadvantages of extensive pre-exposure prophylaxis use. PROSPERO Number: CRD42022356061.
Keywords: Drug resistance mutation; Emtricitabine; HIV; Mutation; Pre-exposure prophylaxis; Tenofovir.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Global Statistics | HIV.gov. https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics. Accessed 04 Apr 2024.
-
- Murchu EO, Marshall L, Teljeur C, Harrington P, Hayes C, Moran P, et al. Oral pre-exposure prophylaxis (PrEP) to prevent HIV: a systematic review and meta-analysis of clinical effectiveness, safety, adherence and risk compensation in all populations. BMJ Open. 2022;12:e048478. doi: 10.1136/bmjopen-2020-048478. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

