Deficiency of SDHC promotes metastasis by reprogramming fatty acid metabolism in colorectal cancer
- PMID: 38844980
- PMCID: PMC11157952
- DOI: 10.1186/s12967-024-05361-x
Deficiency of SDHC promotes metastasis by reprogramming fatty acid metabolism in colorectal cancer
Abstract
Background: Several studies have demonstrated a strong correlation between impaired Succinate dehydrogenase (SDH) function and the advancement of tumors. As a subunit of SDH, succinate dehydrogenase complex subunit C (SDHC) has been revealed to play tumor suppressive roles in several cancers, while its specific role in colorectal cancer (CRC) still needs further investigation.
Methods: Online database were utilized to investigate the expression of SDHC in colorectal cancer and to assess its correlation with patient prognosis. Cell metastasis was assessed using transwell and wound healing assays, while tumor metastasis was studied in a nude mice model in vivo. Drug screening and RNA sequencing were carried out to reveal the tumor suppressor mechanism of SDHC. Triglycerides, neutral lipids and fatty acid oxidation were measured using the Triglyceride Assay Kit, BODIPY 493/503 and Colorimetric Fatty Acid Oxidation Rate Assay Kit, respectively. The expression levels of enzymes involved in fatty acid metabolism and the PI3K/AKT signaling pathway were determined by quantitative real-time PCR and western blot.
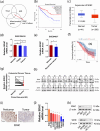
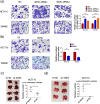
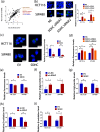
Results: Downregulation of SDHC was found to be closely associated with a poor prognosis in CRC. SDHC knockdown promoted CRC metastasis both in vitro and in vivo. Through drug screening and Gene set enrichment analysis, it was discovered that SDHC downregulation was positively associated with the fatty acid metabolism pathways significantly. The effects of SDHC silencing on metastasis were reversed when fatty acid synthesis was blocked. Subsequent experiments revealed that SDHC silencing activated the PI3K/AKT signaling axis, leading to lipid accumulation by upregulating the expression of aldehyde dehydrogenase 3 family member A2 (ALDH3A2) and reduction of fatty acid oxidation rate by suppressing the expression of acyl-coenzyme A oxidase 1 (ACOX1) and carnitine palmitoyltransferase 1A (CPT1A).
Conclusions: SDHC deficiency could potentially enhance CRC metastasis by modulating the PI3K/AKT pathways and reprogramming lipid metabolism.
Keywords: Colorectal cancer; Fatty acid metabolism; Metastasis; SDHC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Bardella C, Pollard P, Tomlinson I. SDH mutations in cancer. Biochem Biophys Acta. 2011;1807(11):1432–43. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

