A Phase 1, randomized, double-blind, placebo-controlled, single- and multiple-dose escalation study to evaluate the safety and pharmacokinetics/pharmacodynamics of PF-06835375, a C-X-C chemokine receptor type 5 directed antibody, in patients with systemic lupus erythematosus or rheumatoid arthritis
- PMID: 38845046
- PMCID: PMC11155132
- DOI: 10.1186/s13075-024-03337-2
A Phase 1, randomized, double-blind, placebo-controlled, single- and multiple-dose escalation study to evaluate the safety and pharmacokinetics/pharmacodynamics of PF-06835375, a C-X-C chemokine receptor type 5 directed antibody, in patients with systemic lupus erythematosus or rheumatoid arthritis
Abstract
Background: The objective of this study was to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of PF‑06835375, a potent selective afucosyl immunoglobulin G1 antibody targeting C-X-C chemokine receptor type 5 (CXCR5) that potentially depletes B cells, follicular T helper (Tfh) cells, and circulating Tfh-like (cTfh) cells, in patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
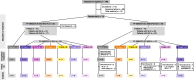
Methods: This first-in-human, multicenter, double-blind, sponsor-open, placebo-controlled Phase 1 study recruited patients aged 18-70 years with SLE or RA. In Part A, patients received single doses of intravenous PF-06835375 (dose range: 0.03-6 mg) or placebo in six sequential single ascending dose (SAD) cohorts. In Part B, patients received repeat doses of subcutaneous PF-06835375 (dose range: 0.3-10 mg) or placebo on Days 1 and 29 in five multiple ascending dose (MAD) cohorts. Tetanus/Diphtheria (Td) and Meningococcal B (MenB/Trumenba™) vaccines were administered at Day 4 (Td and MenB) and Week 8 (MenB only) to assess PF-06835375 functional effects. Endpoints included treatment-emergent adverse events (TEAEs), pharmacokinetic parameters, pharmacodynamic effects on B and cTfh cells, and biomarker counts, vaccine response, and exploratory differential gene expression analysis. Safety, pharmacokinetic, and pharmacodynamic endpoints are summarized descriptively. The change from baseline of B and Tfh cell-specific genes over time was calculated using a prespecified mixed-effects model, with a false discovery rate < 0.05 considered statistically significant.
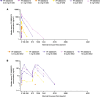
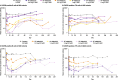
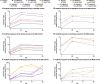
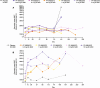
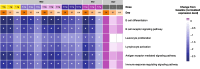
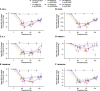
Results: In total, 73 patients were treated (SAD cohorts: SLE, n = 17; RA, n = 14; MAD cohorts: SLE, n = 22; RA, n = 20). Mean age was 53.3 years. Sixty-two (84.9%) patients experienced TEAEs (placebo n = 17; PF-06835375 n = 45); most were mild or moderate. Three (9.7%) patients experienced serious adverse events. Mean t1/2 ranged from 3.4-121.4 h (SAD cohorts) and 162.0-234.0 h (MAD cohorts, Day 29). B and cTfh cell counts generally showed dose-dependent reductions across cohorts (range of mean maximum depletion: 67.3-99.3%/62.4-98.7% [SAD] and 91.1-99.6%/89.5-98.1% [MAD], respectively). B cell-related genes and pathways were significantly downregulated in patients treated with PF-06835375.
Conclusions: These data support further development of PF-06835375 to assess the clinical potential for B and Tfh cell depletion as a treatment for autoimmune diseases.
Trial registration: ClinicalTrials.gov identifier: NCT03334851.
Keywords: B-cell depletion; CXCR5; Efficacy; Follicular helper T cells depletion; PF-06835375; Pharmacodynamics; Pharmacokinetics; Rheumatoid arthritis; Safety; Systemic lupus erythematosus.
© 2024. The Author(s).
Conflict of interest statement
SC is a consultant and investigator for Pfizer Inc. JSB, SK, and MHZ are former employees of Pfizer Inc. VC is an investigator for Pfizer Inc. RL is a consultant for AbbVie, AstraZeneca, and Janssen; investigator for AbbVie, Amgen, AstraZeneca, BMS, Dr. Reddy’s Laboratories, Equillium, GlaxoSmithKline, Idorsia, Janssen, Kangpu, Lilly, Novartis, Pfizer, RemeGen, SunPharm, and Viela Bio; and speaker for AbbVie and GlaxoSmithKline. MV is an employee of and shareholder in Pfizer Inc. SG, LX, MS, CH, SL, MS, AS, ES, EP, DAM, and MC are employees of Pfizer Inc.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

