Nonclinical immunogenicity risk assessment for knobs-into-holes bispecific IgG1 antibodies
- PMID: 38845069
- PMCID: PMC11164226
- DOI: 10.1080/19420862.2024.2362789
Nonclinical immunogenicity risk assessment for knobs-into-holes bispecific IgG1 antibodies
Abstract
Bispecific antibodies, including bispecific IgG, are emerging as an important new class of antibody therapeutics. As a result, we, as well as others, have developed engineering strategies designed to facilitate the efficient production of bispecific IgG for clinical development. For example, we have extensively used knobs-into-holes (KIH) mutations to facilitate the heterodimerization of antibody heavy chains and more recently Fab mutations to promote cognate heavy/light chain pairing for efficient in vivo assembly of bispecific IgG in single host cells. A panel of related monospecific and bispecific IgG1 antibodies was constructed and assessed for immunogenicity risk by comparison with benchmark antibodies with known low (Avastin and Herceptin) or high (bococizumab and ATR-107) clinical incidence of anti-drug antibodies. Assay methods used include dendritic cell internalization, T cell proliferation, and T cell epitope identification by in silico prediction and MHC-associated peptide proteomics. Data from each method were considered independently and then together for an overall integrated immunogenicity risk assessment. In toto, these data suggest that the KIH mutations and in vitro assembly of half antibodies do not represent a major risk for immunogenicity of bispecific IgG1, nor do the Fab mutations used for efficient in vivo assembly of bispecifics in single host cells. Comparable or slightly higher immunogenicity risk assessment data were obtained for research-grade preparations of trastuzumab and bevacizumab versus Herceptin and Avastin, respectively. These data provide experimental support for the common practice of using research-grade preparations of IgG1 as surrogates for immunogenicity risk assessment of their corresponding pharmaceutical counterparts.
Keywords: Anti-drug antibodies; T cell epitope; T cell proliferation; bispecific antibody; ex vivo T cell assay; immunogenicity; in silico prediction; knobs-into-holes.
Conflict of interest statement
All authors are current or former Genentech employees which develops and commercializes therapeutics including monospecific and bispecific antibodies.
Figures
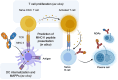
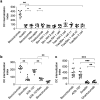
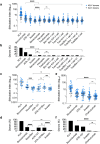


References
-
- The Antibody Society. Therapeutic monoclonal antibodies approved or in regulatory review. [accessed 2024 May 31]. www.antibodysociety.org/antibody-therapeutics-product-data.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
