Characteristics of a cost-effective blood test for colorectal cancer screening
- PMID: 38845072
- PMCID: PMC11461157
- DOI: 10.1093/jnci/djae124
Characteristics of a cost-effective blood test for colorectal cancer screening
Abstract
Background: Blood-based biomarker tests can potentially change the landscape of colorectal cancer (CRC) screening. We characterize the conditions under which blood test screening would be as effective and cost-effective as annual fecal immunochemical testing or decennial colonoscopy.
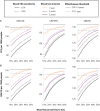
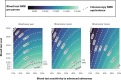
Methods: We used the 3 Cancer Information and Surveillance Modeling Network-Colon models to compare scenarios of no screening, annual fecal immunochemical testing, decennial colonoscopy, and a blood test meeting Centers for Medicare & Medicaid (CMS) coverage criteria (74% CRC sensitivity and 90% specificity). We varied the sensitivity to detect CRC (74%-92%), advanced adenomas (10%-50%), screening interval (1-3 years), and test cost ($25-$500). Primary outcomes included quality-adjusted life-years (QALY) gained from screening and costs for a US average-risk cohort of individuals aged 45 years.
Results: Annual fecal immunochemical testing yielded 125-163 QALY gained per 1000 at a cost of $3811-$5384 per person, whereas colonoscopy yielded 132-177 QALY gained at a cost of $5375-$7031 per person. A blood test with 92% CRC sensitivity and 50% advanced adenoma sensitivity yielded 117-162 QALY gained if used every 3 years and 133-173 QALY gained if used every year but would not be cost-effective if priced above $125 per test. If used every 3 years, a $500 blood test only meeting CMS coverage criteria yielded 83-116 QALY gained at a cost of $8559-$9413 per person.
Conclusion: Blood tests that only meet CMS coverage requirements should not be recommended to patients who would otherwise undergo screening by colonoscopy or fecal immunochemical testing because of lower benefit. Blood tests need higher advanced adenoma sensitivity (above 40%) and lower costs (below $125) to be cost-effective.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- American Cancer Society. Key Statistics for Colorectal Cancer. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statis.... Accessed December 4, 2023.
-
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544-573. doi: 10.1002/cncr.24760 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

