Endovascular treatment to improve outcomes for medium vessel occlusions: The ESCAPE-MeVO trial
- PMID: 38845180
- PMCID: PMC11528926
- DOI: 10.1177/17474930241262642
Endovascular treatment to improve outcomes for medium vessel occlusions: The ESCAPE-MeVO trial
Abstract
Rationale: Clinical outcomes in acute ischemic stroke due to medium vessel occlusion (MeVO) are often poor when treated with best medical management. Data from non-randomized studies suggest that endovascular treatment (EVT) may improve outcomes in MeVO stroke, but randomized data on potential benefits and risks are hitherto lacking. Thus, there is insufficient evidence to guide EVT decision-making in MeVO stroke.
Aims: The primary aim of the ESCAPE-MeVO trial is to demonstrate that acute, rapid EVT in patients with acute ischemic stroke due to MeVO results in better clinical outcomes compared to best medical management. Secondary outcomes are to demonstrate the safety of EVT, its impact on self-reported health-related quality of life, and cost-effectiveness.
Sample size estimates: Based on previously published data, we estimate a sample size of 500 subjects to achieve a power of 85% with a two-sided alpha of 0.05. To account for potential loss to follow-up, 530 subjects will be recruited.
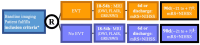
Methods and design: ESCAPE-MeVO is a multicenter, prospective, randomized, open-label study with blinded endpoint evaluation (PROBE design), clinicaltrials.gov: NCT05151172. Subjects with acute ischemic stroke due to MeVO meeting the trial eligibility criteria will be allocated in a 1:1 ratio to best medical care plus EVT versus best medical care only. Patients will be screened only at comprehensive stroke centers to determine if they are eligible for the trial, regardless of whether they were previously treated at a primary care center. Key eligibility criteria are (1) acute ischemic stroke due to MeVO that is clinically and technically eligible for EVT, (2) last-known well within the last 12 h, (3) National Institutes of Health Stroke Scale > 5 or 3-5 with disabling deficit, (4) high likelihood of salvageable tissue on non-invasive neuroimaging.
Study outcomes: The primary outcome is the modified Rankin scale 90 days after randomization (shift analysis), whereby modified Rankin Score 5 and 6 will be collapsed into one category. Secondary outcomes include dichotomizations of the modified Rankin Score at 90 days, 24 h National Institutes of Health Stroke Score, difference between 24 h and baseline National Institutes of Health Stroke Score, mortality at 90 days, health-related quality of life (EQ-5D-5 L), Lawton scale of instrumental activities of daily living score, reperfusion quality (MeVO expanded Thrombolysis in Cerebral Infarction Score) and infarct volume at 24 h, and cost-effectiveness of endovascular recanalization. Safety outcomes include symptomatic and asymptomatic intracranial hemorrhage and procedural complications.
Discussion: The ESCAPE-MeVO trial will demonstrate the effect of endovascular thrombectomy in addition to best medical management vis-à-vis best medical management in patients with acute ischemic stroke due to MeVO and provide data for evidence-based treatment decision-making in acute MeVO stroke.
Data access statement: The raw data discussed in this mansucript will be made available by the corresponding author upon reasonable request.
Keywords: Medium vessel occlusion; acute ischemic stroke; endovascular treatment; mechanical thrombectomy.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Mayank Goyal and Michael Hill report research grants from Medtronic.
Figures
References
-
- Ospel JM, Menon BK, Demchuk AM, et al.. Clinical course of acute ischemic stroke due to medium vessel occlusion with and without intravenous alteplase treatment. Stroke 2020; 51: 3232–3240. - PubMed
-
- Ospel JM, Goyal M. A review of endovascular treatment for medium vessel occlusion stroke. J Neurointerv Surg 2021; 13: 623–630. - PubMed
-
- Ospel JM, Nguyen TN, Jadhav AP, et al.. Endovascular treatment of medium vessel occlusion stroke. Stroke 2024; 55: 769–778. - PubMed
-
- Limaye K, Koo AB, De Havenon A, et al.. Safety and efficacy of MCA-M2 thrombectomy in delayed time window: a propensity score analysis from the STAR registry. Stroke 2023; 3: e000664.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

