Cost burden of cirrhosis and liver disease progression in metabolic dysfunction-associated steatohepatitis: A US cohort study
- PMID: 38845444
- PMCID: PMC11365567
- DOI: 10.18553/jmcp.2024.24069
Cost burden of cirrhosis and liver disease progression in metabolic dysfunction-associated steatohepatitis: A US cohort study
Abstract
Background: Metabolic dysfunction-associated steatohepatitis (MASH), formerly nonalcoholic steatohepatitis, is characterized by fat accumulation and inflammation of the liver and may result in progression to cirrhosis and liver-related events.
Objective: To characterize the impact of cirrhosis and progression to liver-related events on costs and health care resource use (HCRU) among MASH patients in the United States.
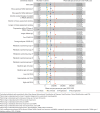
Methods: The study cohort included patients with diagnosed nonalcoholic steatohepatitis (International Classification of Diseases, Tenth Revision, Clinical Modification code K75.81) in Optum's deidentified Clinformatics Data Mart Database (October 2015 to December 2022) and were stratified by baseline cirrhosis status. Among those without cirrhosis at baseline, patients were further stratified by status of progression to cirrhosis during follow-up. Total HCRU and costs per-person per-year (PPPY) were estimated and compared descriptively between the cohorts. In addition, gamma generalized linear models were used to compare costs PPPY between those with vs without cirrhosis at baseline, as well as with vs without progression during follow-up, while adjusting for baseline patient and disease characteristics. Annual costs per person were also longitudinally modeled using gamma generalized linear mixed models to understand longitudinal changes in costs PPPY while accounting for time correlations within individual patients. Lastly, a series of sensitivity analyses were conducted to assess the impact of study design features and clinical variations of total costs PPPY.
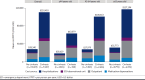
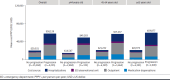
Results: A total of 28,576 adults were included, and 9,157 (32.0%) had baseline cirrhosis; of the 19,419 without baseline cirrhosis, a total of 4,235 (21.8%) progressed over follow-up. Mean (SD) HCRU and costs PPPY were higher among patients with cirrhosis ($110,403 [$226,037]) than without ($28,340 [$61,472]; P < 0.01) and among those with progression ($58,128 [$102,626]) than without ($20,031 [$39,740]; P < 0.01). Costs remained significantly greater when adjusted for covariates, with a risk ratio (95% CI) of 1.99 (1.89-2.09) when comparing with vs without baseline cirrhosis and 2.28 (2.15-2.42) when comparing with vs without progression over follow-up. Costs increased with each subsequent year, to 21% by year 6 among those with cirrhosis at baseline and 49% among those without baseline cirrhosis who progressed.
Conclusions: The financial burden of MASH is substantial and significantly greater among those with cirrhosis or disease progression. Although patients without cirrhosis incur lower burden, the increase over time is greater and associated with progression. Therapies that slow progression may help alleviate the financial burden, and strategies are needed to identify patients with MASH at risk of progressing to cirrhosis.
Conflict of interest statement
This study was supported by Madrigal Pharmaceuticals, Inc. Grant number: not applicable.
Dr Charlton has received advisory and consulting honoraria from Madrigal Pharmaceuticals, Inc., Novo Nordisk, Cytodyn, Terns, Alnylam, AMR, Glympse, Northsea, Sagmimet, Genentech, and Merck; he has also received research grants from Pfizer, unrelated to this work.
Figures
Similar articles
-
Estimating the clinical and healthcare burden of metabolic dysfunction-associated steatohepatitis in England: a retrospective cohort study using routinely collected healthcare data from 2011 to 2020.BMJ Open. 2025 Apr 23;15(4):e095761. doi: 10.1136/bmjopen-2024-095761. BMJ Open. 2025. PMID: 40268491 Free PMC article.
-
Evaluating the burden of illness of metabolic dysfunction-associated steatohepatitis in a large managed care population: The ETHEREAL Study.J Manag Care Spec Pharm. 2024 Dec;30(12):1414-1430. doi: 10.18553/jmcp.2024.24106. Epub 2024 Sep 27. J Manag Care Spec Pharm. 2024. PMID: 39331041 Free PMC article.
-
The clinical and economic burdens of metabolic dysfunction-associated steatohepatitis.J Med Econ. 2024 Jan-Dec;27(1):919-930. doi: 10.1080/13696998.2024.2374642. Epub 2024 Jul 17. J Med Econ. 2024. PMID: 38953706
-
The Economic Burden of Non-Alcoholic Steatohepatitis: A Systematic Review.Pharmacoeconomics. 2022 Aug;40(8):751-776. doi: 10.1007/s40273-022-01140-y. Epub 2022 Jul 5. Pharmacoeconomics. 2022. PMID: 35789987 Free PMC article.
-
Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease.Hepatology. 2018 Jan;67(1):123-133. doi: 10.1002/hep.29466. Epub 2017 Dec 1. Hepatology. 2018. PMID: 28802062 Free PMC article. Review.
Cited by
-
Costs associated with nonalcoholic steatohepatitis disease progression in Medicare patients: a retrospective cohort study.J Comp Eff Res. 2024 Dec;13(12):e240096. doi: 10.57264/cer-2024-0096. Epub 2024 Nov 22. J Comp Eff Res. 2024. PMID: 39576038 Free PMC article.
-
Estimation of the Eligible Population For Resmetirom Among Adults in the United States for Treatment of Non-Cirrhotic NASH with Moderate-to-Advanced Liver Fibrosis.Adv Ther. 2024 Nov;41(11):4172-4190. doi: 10.1007/s12325-024-02989-5. Epub 2024 Sep 18. Adv Ther. 2024. PMID: 39292422 Free PMC article.
-
Estimating the clinical and healthcare burden of metabolic dysfunction-associated steatohepatitis in England: a retrospective cohort study using routinely collected healthcare data from 2011 to 2020.BMJ Open. 2025 Apr 23;15(4):e095761. doi: 10.1136/bmjopen-2024-095761. BMJ Open. 2025. PMID: 40268491 Free PMC article.
-
Survival and Cost-Effectiveness of Bariatric Surgery Among Patients With Obesity and Cirrhosis.JAMA Surg. 2025 Jun 1;160(6):645-655. doi: 10.1001/jamasurg.2025.0490. JAMA Surg. 2025. PMID: 40172871
-
Progression from Non-alcoholic Steatohepatitis to Advanced Liver Diseases and Mortality Among Medicare Patients.Adv Ther. 2024 Nov;41(11):4335-4355. doi: 10.1007/s12325-024-02979-7. Epub 2024 Sep 24. Adv Ther. 2024. PMID: 39316292 Free PMC article.
References
-
- Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323(12):1175-83. doi:10.1001/jama.2020.2298 - PubMed
-
- Estes C, Anstee QM, Arias-Loste MT, et al. . Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896-904. doi:10.1016/j.jhep.2018.05.036 - PubMed
-
- National Institute of Diabetes and Digestive and Kidney Diseases. Symptoms & causes of NAFLD & NASH. 2021. Accessed August 22, 2023. https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/sy...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

