Experimental Verification of Erchen Decoction Plus Huiyanzhuyu Decoction in the Treatment of Laryngeal Squamous Cell Carcinoma Based on Network Pharmacology
- PMID: 38845538
- PMCID: PMC11159533
- DOI: 10.1177/15347354241259182
Experimental Verification of Erchen Decoction Plus Huiyanzhuyu Decoction in the Treatment of Laryngeal Squamous Cell Carcinoma Based on Network Pharmacology
Abstract
Background: The prescription of Chinese herbal medicine (CHM) consists of multiple herbs that exhibit synergistic effects due to the presence of multiple components targeting various pathways. In clinical practice, the combination of Erchen decoction and Huiyanzhuyu decoction (EHD) has shown promising outcomes in treating patients with laryngeal squamous cell carcinoma (LSCC). However, the underlying mechanism by which EHD exerts its therapeutic effects in LSCC remains unknown.
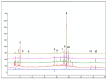
Methods: Online databases were utilized for the analysis and prediction of the active constituents, targets, and key pathways associated with EHD in the treatment of LSCC. The protein-protein interaction (PPI) network of common targets was constructed and visualized using Cytoscape 3.8.1 software. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed to investigate the functional roles of core targets within the PPI network. Protein clustering was conducted utilizing the MCODE plug-in. The obtained results highlight the principal targets and pathways involved. Subsequently, clinical samples were collected to validate alterations in the levels of these main targets through Western blotting (WB) and immunohistochemistry (IHC). Furthermore, both in vivo and in vitro experiments were conducted to investigate the therapeutic effects of EHD on healing LSCC and elucidate its underlying mechanism. Additionally, to ensure experimental reliability and reproducibility, quality control measures utilizing HPLC were implemented for EHD herbal medicine.
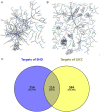
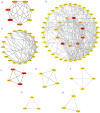
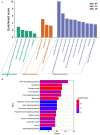
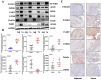
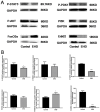
Results: The retrieval and analysis of databases in EHD medicine and LSCC disease yielded a total of 116 overlapping targets. The MCODE plug-in methods were utilized to acquire 8 distinct protein clusters through protein clustering. The findings indicated that both the first and second clusters exhibited a size greater than 6 scores, with key genes PI3K and ErbB occupying central positions, while the third and fourth clusters were associated with proteins in the PI3K, STAT3, and Foxo pathways. GO functional analysis reported that these targets had associations mainly with the pathway of p53 mediated DNA damage and negative regulation of cell cycle in terms of biological function; the death-induced signaling complex in terms of cell function; transcription factor binding and protein kinase activity in terms of molecular function. The KEGG enrichment analysis demonstrated that these targets were correlated with several signaling pathways, including PI3K-Akt, FoxO, and ErbB2 signaling pathway. On one hand, we observed higher levels of key genes such as P-STAT3, P-PDK1, P-Akt, PI3K, and ErbB2 in LSCC tumor tissues compared to adjacent tissues. Conversely, FOXO3a expression was lower in LSCC tumor tissues. On the other hand, the key genes mentioned above were also highly expressed in both LSCC xenograft nude mice tumors and LSCC cell lines, while FOXO3a was underexpressed. In LSCC xenograft nude mice models, EHD treatment resulted in downregulation of P-STAT3, P-PDK1, PI3K, P-AKT, and ErbB2 protein levels but upregulated FOXO3a protein level. EHD also affected the levels of P-STAT3, P-PDK1, PI3K, P-AKT, FOXO3a, and ErbB2 proteins in vitro: it inhibited P-STAT3, P-AKT, and ErbB2, while promoting FOXO3a; however, it had no effect on PDK1 protein. In addition, HPLC identified twelve compounds accounting for more than 30% within EHD. The findings from this study can serve as valuable guidance for future experimental investigations.
Conclusion: The possible mechanism of EHD medicine action on LSCC disease is speculated to be closely associated with the ErbB2/PI3K/AKT/FOXO3a signaling pathway.
Keywords: Chinese herbal medicine; ErbB2/PI3K/AKT/Foxo3a signaling pathway; Erchen decoction plus Huiyanzhuyu decoction; laryngeal squamous cell carcinoma; phlegm coagulation and blood stasis syndrome.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Erchen decoction plus huiyanzhuyu decoction inhibits the cell cycle, migration and invasion and induces the apoptosis of laryngeal squamous cell carcinoma cells.J Ethnopharmacol. 2020 Jun 28;256:112638. doi: 10.1016/j.jep.2020.112638. Epub 2020 Jan 31. J Ethnopharmacol. 2020. PMID: 32007633
-
Erchen Plus Huiyanzhuyu Decoction Inhibits the Growth of Laryngeal Carcinoma in a Mouse Model of Phlegm-Coagulation-Blood-Stasis Syndrome via the STAT3/Cyclin D1 Pathway.Evid Based Complement Alternat Med. 2020 Apr 22;2020:2803496. doi: 10.1155/2020/2803496. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32382281 Free PMC article.
-
A network pharmacology approach and experimental validation to investigate the anticancer mechanism and potential active targets of ethanol extract of Wei-Tong-Xin against colorectal cancer through induction of apoptosis via PI3K/AKT signaling pathway.J Ethnopharmacol. 2023 Mar 1;303:115933. doi: 10.1016/j.jep.2022.115933. Epub 2022 Nov 18. J Ethnopharmacol. 2023. PMID: 36403742
-
Xuebijing and somatostatin against acute pancreatitis: A systematic review and network pharmacology.Medicine (Baltimore). 2024 Dec 13;103(50):e40964. doi: 10.1097/MD.0000000000040964. Medicine (Baltimore). 2024. PMID: 39686429 Free PMC article.
-
Luteolin: A Comprehensive and Visualized Analysis of Research Hotspots and Its Antitumor Mechanisms.Am J Chin Med. 2024;52(8):2377-2401. doi: 10.1142/S0192415X24500903. Epub 2024 Dec 16. Am J Chin Med. 2024. PMID: 39686791 Review.
References
-
- Tan X, Luo Q, Zhou S, et al.. Erchen plus Huiyanzhuyu decoction inhibits the growth of laryngeal carcinoma in a mouse model of phlegm-coagulation-blood-stasis syndrome via the STAT3/Cyclin D1 pathway. Evid Based Complement Alternat Med. 2020;2020:2803496. doi:10.1155/2020/2803496 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

