Unveiling brain disorders using liquid biopsy and Raman spectroscopy
- PMID: 38845582
- PMCID: PMC11290551
- DOI: 10.1039/d4nr01413h
Unveiling brain disorders using liquid biopsy and Raman spectroscopy
Abstract
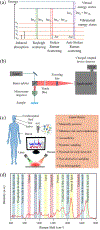
Brain disorders, including neurodegenerative diseases (NDs) and traumatic brain injury (TBI), present significant challenges in early diagnosis and intervention. Conventional imaging modalities, while valuable, lack the molecular specificity necessary for precise disease characterization. Compared to the study of conventional brain tissues, liquid biopsy, which focuses on blood, tear, saliva, and cerebrospinal fluid (CSF), also unveils a myriad of underlying molecular processes, providing abundant predictive clinical information. In addition, liquid biopsy is minimally- to non-invasive, and highly repeatable, offering the potential for continuous monitoring. Raman spectroscopy (RS), with its ability to provide rich molecular information and cost-effectiveness, holds great potential for transformative advancements in early detection and understanding the biochemical changes associated with NDs and TBI. Recent developments in Raman enhancement technologies and advanced data analysis methods have enhanced the applicability of RS in probing the intricate molecular signatures within biological fluids, offering new insights into disease pathology. This review explores the growing role of RS as a promising and emerging tool for disease diagnosis in brain disorders, particularly through the analysis of liquid biopsy. It discusses the current landscape and future prospects of RS in the diagnosis of brain disorders, highlighting its potential as a non-invasive and molecularly specific diagnostic tool.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures









References
-
- Payne TD, Moody AS, Wood AL, Pimiento PA, Elliott JC and Sharma B, Analyst, 2020, 145, 3461–3480. - PubMed
-
- Hrubešová K, Fousková M, Habartová L, Fišar Z, Jirák R, Raboch J. and SETNIčKA V, Clinical Biochemistry, 2019, 72, 39–51. - PubMed
-
- Paraskevaidi M, Martin-Hirsch PL and Martin FL, ACS chemical neuroscience, 2018, 9, 446–461. - PubMed
-
- Alzheimer’s Disease Facts and Figures, https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf, (accessed 03/20/2024).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

