Ethyl 2-[(2-oxo-2 H-chromen-6-yl)-oxy]acetate
- PMID: 38845711
- PMCID: PMC11151299
- DOI: 10.1107/S2056989024004729
Ethyl 2-[(2-oxo-2 H-chromen-6-yl)-oxy]acetate
Abstract
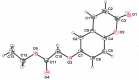
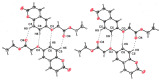
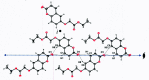
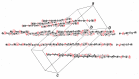
Ethyl 2-[(2-oxo-2H-chromen-6-yl)-oxy]acetate, C13H12O5, a member of the pharmacologically important class of coumarins, crystallizes in the monoclinic C2/c space group in the form of sheets, within which mol-ecules are related by inversion centers and 21 axes. Multiple C-H⋯O weak hydrogen-bonding inter-actions reinforce this pattern. The planes of these sheets are oriented in the approximate direction of the ac face diagonal. Inter-sheet inter-actions are a combination of coumarin system π-π stacking and additional C-H⋯O weak hydrogen bonds between ethyl acet-oxy groups.
Keywords: chromen-2-one; coumarin; crystal structure; sheet structure; weak C—H⋯O H-bonding; π–π stacking.
© Goyal et al. 2024.
Figures







References
-
- Bandaru, S. S. M., Chrysochos, N. & Schulzke, C. (2019). CSD Communication (CCDC 1842722). CCDC, Cambridge, England.
-
- Baures, P. W., Rush, J. R., Schroeder, S. D. & Beatty, A. M. (2002). Cryst. Growth Des. 2, 107–110.
-
- Bruker (2021). APEX4 and SAINT. Bruker AXS Inc., Madison, Wisncosin, USA.
-
- Chung, D. D. L. (2002). J. Mater. Sci. 37, 1–15.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
