Stable overexpression of native and artificial miRNAs for the production of differentially fucosylated antibodies in CHO cells
- PMID: 38845814
- PMCID: PMC11151017
- DOI: 10.1002/elsc.202300234
Stable overexpression of native and artificial miRNAs for the production of differentially fucosylated antibodies in CHO cells
Abstract
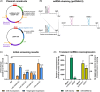
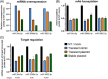
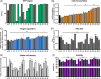
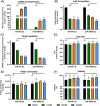
Cell engineering strategies typically rely on energy-consuming overexpression of genes or radical gene-knock out. Both strategies are not particularly convenient for the generation of slightly modulated phenotypes, as needed in biosimilar development of for example differentially fucosylated monoclonal antibodies (mAbs). Recently, transiently transfected small noncoding microRNAs (miRNAs), known to be regulators of entire gene networks, have emerged as potent fucosylation modulators in Chinese hamster ovary (CHO) production cells. Here, we demonstrate the applicability of stable miRNA overexpression in CHO production cells to adjust the fucosylation pattern of mAbs as a model phenotype. For this purpose, we applied a miRNA chaining strategy to achieve adjustability of fucosylation in stable cell pools. In addition, we were able to implement recently developed artificial miRNAs (amiRNAs) based on native miRNA sequences into a stable CHO expression system to even further fine-tune fucosylation regulation. Our results demonstrate the potential of miRNAs as a versatile tool to control mAb fucosylation in CHO production cells without adverse side effects on important process parameters.
Keywords: CHO; biopharmaceuticals; biosimilar; fucosylation engineering; microRNA.
© 2024 The Authors. Engineering in Life Sciences published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A novel system for glycosylation engineering by natural and artificial miRNAs.Metab Eng. 2023 May;77:53-63. doi: 10.1016/j.ymben.2023.03.004. Epub 2023 Mar 9. Metab Eng. 2023. PMID: 36906118
-
MiRNA Chaining for Efficient Stable Overexpression to Improve Protein Quantity and Quality in CHO Cells.Methods Mol Biol. 2025;2853:85-101. doi: 10.1007/978-1-0716-4104-0_7. Methods Mol Biol. 2025. PMID: 39460916
-
Stable miRNA overexpression in human CAP cells: Engineering alternative production systems for advanced manufacturing of biologics using miR-136 and miR-3074.Biotechnol Bioeng. 2018 Aug;115(8):2027-2038. doi: 10.1002/bit.26715. Epub 2018 May 3. Biotechnol Bioeng. 2018. PMID: 29665036
-
Enhancing recombinant antibody yield in Chinese hamster ovary cells.Tzu Chi Med J. 2024 May 24;36(3):240-250. doi: 10.4103/tcmj.tcmj_315_23. eCollection 2024 Jul-Sep. Tzu Chi Med J. 2024. PMID: 38993821 Free PMC article. Review.
-
The Effect of microRNA on the Production of Recombinant Protein in CHO Cells and its Mechanism.Front Bioeng Biotechnol. 2022 Mar 21;10:832065. doi: 10.3389/fbioe.2022.832065. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35387297 Free PMC article. Review.
References
-
- Yamane‐Ohnuki N, Kinoshita S, Inoue‐Urakubo M, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody‐dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614‐622. doi:10.1002/bit.20151 - DOI - PubMed
LinkOut - more resources
Full Text Sources
