Notoginsenoside R1 Ameliorate High-Fat-Diet and Vitamin D3-Induced Atherosclerosis via Alleviating Inflammatory Response, Inhibiting Endothelial Dysfunction, and Regulating Gut Microbiota
- PMID: 38845851
- PMCID: PMC11155380
- DOI: 10.2147/DDDT.S451565
Notoginsenoside R1 Ameliorate High-Fat-Diet and Vitamin D3-Induced Atherosclerosis via Alleviating Inflammatory Response, Inhibiting Endothelial Dysfunction, and Regulating Gut Microbiota
Abstract
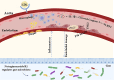
Aim: Natural medicines possess significant research and application value in the field of atherosclerosis (AS) treatment. The study was performed to investigate the impacts of a natural drug component, notoginsenoside R1, on the development of atherosclerosis (AS) and the potential mechanisms.
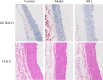
Methods: Rats induced with AS by a high-fat-diet and vitamin D3 were treated with notoginsenoside R1 for six weeks. The ameliorative effect of NR1 on AS rats was assessed by detecting pathological changes in the abdominal aorta, biochemical indices in serum and protein expression in the abdominal aorta, as well as by analysing the gut microbiota.
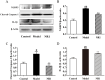
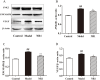
Results: The NR1 group exhibited a noticeable reduction in plaque pathology. Notoginsenoside R1 can significantly improve serum lipid profiles, encompassing TG, TC, LDL, ox-LDL, and HDL. Simultaneously, IL-6, IL-33, TNF-α, and IL-1β levels are decreased by notoginsenoside R1 in lowering inflammatory elements. Notoginsenoside R1 can suppress the secretion of VCAM-1 and ICAM-1, as well as enhance the levels of plasma NO and eNOS. Furthermore, notoginsenoside R1 inhibits the NLRP3/Cleaved Caspase-1/IL-1β inflammatory pathway and reduces the expression of the JNK2/P38 MAPK/VEGF endothelial damage pathway. Fecal analysis showed that notoginsenoside R1 remodeled the gut microbiota of AS rats by decreasing the count of pathogenic bacteria (such as Firmicutes and Proteobacteria) and increasing the quantity of probiotic bacteria (such as Bacteroidetes).
Conclusion: Notoginsenoside R1, due to its unique anti-inflammatory properties, may potentially prevent the progression of atherosclerosis. This mechanism helps protect the vascular endothelium from damage, while also regulating the imbalance of intestinal microbiota, thereby maintaining the overall health of the body.
Keywords: endothelial damage; gut microbiota; inflammation; notoginsenoside R1.
© 2024 Ma et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Inflammation inhibition and gut microbiota regulation by TSG to combat atherosclerosis in ApoE-/- mice.J Ethnopharmacol. 2020 Jan 30;247:112232. doi: 10.1016/j.jep.2019.112232. Epub 2019 Oct 10. J Ethnopharmacol. 2020. PMID: 31606534
-
Edaravone Ameliorate Inflammation in Vitamin D3 and High Fat Diet Induced Atherosclerosis in Rat via Alteration of Inflammatory Pathway and Gut Microbiota.Chem Biol Drug Des. 2024 Nov;104(5):e70019. doi: 10.1111/cbdd.70019. Chem Biol Drug Des. 2024. PMID: 39572910
-
Weizmannia coagulans JA845 improves atherosclerosis induced by vitamin D3 and high-fat diet in rats through modulating lipid metabolism, oxidative stress, and endothelial vascular injury.J Appl Microbiol. 2023 Aug 1;134(8):lxad165. doi: 10.1093/jambio/lxad165. J Appl Microbiol. 2023. PMID: 37516440
-
Astragaloside IV Mediates the PI3K/Akt/mTOR Pathway to Alleviate Injury and Modulate the Composition of Intestinal Flora in ApoE-/- Atherosclerosis Model Rats.Discov Med. 2024 May;36(184):1070-1079. doi: 10.24976/Discov.Med.202436184.99. Discov Med. 2024. PMID: 38798265
-
Anti-atherosclerosis of oligomeric proanthocyanidins from Rhodiola rosea on rat model via hypolipemic, antioxidant, anti-inflammatory activities together with regulation of endothelial function.Phytomedicine. 2018 Dec 1;51:171-180. doi: 10.1016/j.phymed.2018.10.002. Epub 2018 Oct 4. Phytomedicine. 2018. PMID: 30466614
Cited by
-
Gut microbiota regulate atherosclerosis via the gut-vascular axis: a scoping review of mechanisms and therapeutic interventions.Front Microbiol. 2025 Aug 8;16:1606309. doi: 10.3389/fmicb.2025.1606309. eCollection 2025. Front Microbiol. 2025. PMID: 40862134 Free PMC article.
-
The integration of spear and shield: a panoramic analysis of the blood circulation-promoting and hemostatic effects of Panax notoginseng.Chin Med. 2025 May 29;20(1):79. doi: 10.1186/s13020-025-01100-6. Chin Med. 2025. PMID: 40442777 Free PMC article. Review.
-
The NLRP3 inflammasome: a therapeutic target of phytochemicals in treating atherosclerosis (a systematic review).Front Immunol. 2025 May 15;16:1568722. doi: 10.3389/fimmu.2025.1568722. eCollection 2025. Front Immunol. 2025. PMID: 40443656 Free PMC article.
-
Heqi San alleviates diabetic atherosclerosis and alters serum metabolic profiles in rats.BMC Cardiovasc Disord. 2025 Aug 18;25(1):610. doi: 10.1186/s12872-025-05012-z. BMC Cardiovasc Disord. 2025. PMID: 40826035 Free PMC article.
-
Synergistic effects of notoginsenoside R1 and saikosaponin B2 in atherosclerosis: A novel approach targeting PI3K/AKT/mTOR pathway and macrophage autophagy.PLoS One. 2025 Jun 27;20(6):e0326687. doi: 10.1371/journal.pone.0326687. eCollection 2025. PLoS One. 2025. PMID: 40577270 Free PMC article.
References
-
- Ma L, Zhao Z, Zhao Y, Gao Y, Zhao L, Li S. Weizmannia coagulans JA845 improves atherosclerosis induced by vitamin D3 and high-fat diet in rats through modulating lipid metabolism, oxidative stress, and endothelial vascular injury. J Appl Microbiol. 2023;134(8):lxad165. doi:10.1093/jambio/lxad165 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

