Unlocking fungal quorum sensing: Oxylipins and yeast interactions enhance secondary metabolism in monascus
- PMID: 38845857
- PMCID: PMC11154204
- DOI: 10.1016/j.heliyon.2024.e31619
Unlocking fungal quorum sensing: Oxylipins and yeast interactions enhance secondary metabolism in monascus
Abstract
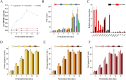
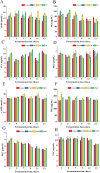
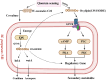
Exploring the symbiotic potential between fungal and yeast species, this study investigates the co-cultivation dynamics of Monascus, a prolific producer of pharmacologically relevant secondary metabolites, and Wickerhamomyce anomalous. The collaborative interaction between these microorganisms catalyzed a substantial elevation in the biosynthesis of secondary metabolites, prominently Monacolin K and natural pigments. Central to our discoveries was the identification and enhanced production of oxylipins (13S-hydroxyoctadecadienoic acid,13S-HODE), putative quorum-sensing molecules, within the co-culture environment. Augmentation with exogenous oxylipins not only boosted Monacolin K production by over half but also mirrored morphological adaptations in Monascus, affecting both spores and mycelial structures. This augmentation was paralleled by a significant upregulation in the transcriptional activity of genes integral to the Monacolin K biosynthetic pathway, as well as genes implicated in pigment and spore formation. Through elucidating the interconnected roles of quorum sensing, G-protein-coupled receptors, and the G-protein-mediate signaling pathway, this study provides a comprehensive view of the molecular underpinnings facilitating these metabolic enhancements. Collectively, our findings illuminate the profound influence of Wickerhamomyces anomalous co-culture on Monascus purpureus, advocating for oxylipins as a pivotal quorum-sensing mechanism driving the observed symbiotic benefits.
Keywords: Monascus purpureus; Oxylipins; Quorum sensing; Secondary metabolites; Wickerhamomyces anomalous.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Linoleic acid functions as a quorum-sensing molecule in Monascus purpureus-Saccharomyces cerevisiae co-culture.Yeast. 2023 Jan;40(1):42-52. doi: 10.1002/yea.3831. Epub 2022 Dec 20. Yeast. 2023. PMID: 36514193
-
Overexpression of global regulator LaeA increases secondary metabolite production in Monascus purpureus.Appl Microbiol Biotechnol. 2020 Apr;104(7):3049-3060. doi: 10.1007/s00253-020-10379-4. Epub 2020 Feb 11. Appl Microbiol Biotechnol. 2020. PMID: 32043189
-
Divergence of metabolites in three phylogenetically close Monascus species (M. pilosus, M. ruber, and M. purpureus) based on secondary metabolite biosynthetic gene clusters.BMC Genomics. 2020 Oct 1;21(1):679. doi: 10.1186/s12864-020-06864-9. BMC Genomics. 2020. PMID: 32998685 Free PMC article.
-
The regulatory functions of oxylipins in fungi: A review.J Basic Microbiol. 2023 Oct;63(10):1073-1084. doi: 10.1002/jobm.202200721. Epub 2023 Jun 26. J Basic Microbiol. 2023. PMID: 37357952 Review.
-
Monascin and ankaflavin-Biosynthesis from Monascus purpureus, production methods, pharmacological properties: A review.Biotechnol Appl Biochem. 2023 Feb;70(1):137-147. doi: 10.1002/bab.2336. Epub 2022 Apr 11. Biotechnol Appl Biochem. 2023. PMID: 35353924 Review.
Cited by
-
Life on a leaf: the epiphyte to pathogen continuum and interplay in the phyllosphere.BMC Biol. 2024 Aug 7;22(1):168. doi: 10.1186/s12915-024-01967-1. BMC Biol. 2024. PMID: 39113027 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

