Development and clinical evaluation of a real-time multiple cross displacement amplification assay for rapid and sensitive detection of Mycobacterium tuberculosis
- PMID: 38845879
- PMCID: PMC11154602
- DOI: 10.1016/j.heliyon.2024.e31901
Development and clinical evaluation of a real-time multiple cross displacement amplification assay for rapid and sensitive detection of Mycobacterium tuberculosis
Abstract
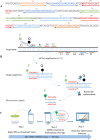
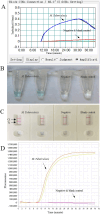
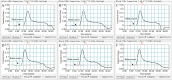
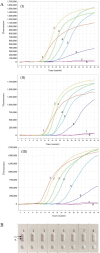
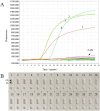
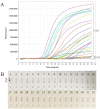
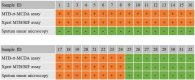
Molecular techniques of nucleic acid testing recommended by the World Health Organization (WHO) for the Mycobacterium tuberculosis (MTB) detection were considered to have the potential access to the accurate tuberculosis (TB) notifications. In this study, a new method, which coupled real-time (rt) fluorescence technique with multiple cross displacement amplification (MCDA), was developed for the rapid, sensitive and specific detection of MTB (termed MTB-rt-MCDA). According to the principle of the rt-MCDA test, a set of ten primers were designed for the MCDA reaction, of which one was engineered with a restrictive endonuclease recognition site, a fluorophore and a quencher for achieving the real-time fluorescence detection. MTB-rt-MCDA test was conducted under the optimized conditions (67 °C, 40 min) on the real-time fluorescence platform. The MTB-rt-MCDA assay accurately identified the MTB strains with no cross reaction with other bacteria. The lowest detectable genomic DNA concentration of the MTB-rt-MCDA assay was 25 fg/μl. We employed the genomic DNA templates extracted from sputum of clinical cases for validating the practical applicability of this assay, and the detection power of the MTB-rt-MCDA assay was comparable to that of the Xpert method and MCDA-based biosensor detection and superior to smear microscope method. The complete process of the MTB-rt-MCDA assay, including rapid extraction of DNA and rt-MCDA test, takes less than 1 h. In conclusion, the presented MTB-rt-MCDA assay provided an effective and simple option for the rapid screening of MTB infection.
Keywords: Lateral flow biosensor; Multiple cross displacement amplification; Mycobacterium tuberculosis; Rapid diagnosis; Real-time.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Two target genes based multiple cross displacement amplification combined with a lateral flow biosensor for the detection of Mycobacterium tuberculosis complex.BMC Microbiol. 2021 Oct 4;21(1):267. doi: 10.1186/s12866-021-02328-6. BMC Microbiol. 2021. PMID: 34607556 Free PMC article.
-
A CRISPR-Cas12a-based platform for ultrasensitive rapid highly specific detection of Mycobacterium tuberculosis in clinical application.Front Cell Infect Microbiol. 2023 May 23;13:1192134. doi: 10.3389/fcimb.2023.1192134. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37287467 Free PMC article.
-
Real-time fluorescent multiple cross displacement amplification for rapid and sensitive Mycoplasma pneumoniae detection.Front Cell Infect Microbiol. 2024 Aug 8;14:1423155. doi: 10.3389/fcimb.2024.1423155. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39176262 Free PMC article.
-
Development and Clinical Validation of Multiple Cross Displacement Amplification Combined With Nanoparticles-Based Biosensor for Detection of Mycobacterium tuberculosis: Preliminary Results.Front Microbiol. 2019 Sep 13;10:2135. doi: 10.3389/fmicb.2019.02135. eCollection 2019. Front Microbiol. 2019. PMID: 31572340 Free PMC article.
-
A diagnostic test that uses isothermal amplification and lateral flow detection sdaA can detect tuberculosis in 60 min.J Appl Microbiol. 2021 Jun;130(6):2102-2110. doi: 10.1111/jam.14902. Epub 2020 Nov 12. J Appl Microbiol. 2021. PMID: 33070404
References
-
- WHO . World Health Organization; 2022. Global Tuberculosis Reports.
-
- WHO . World Health Organization; 2022. Tuberculosis Deaths and Disease Increase during the COVID-19 Pandemic.
-
- Sester M., Giehl C., McNerney R., Kampmann B., Walzl G., Cuchí P., Wingfield C., Lange C., Migliori G.B., Kritski A.L., Meyerhans A. Challenges and perspectives for improved management of HIV/Mycobacterium tuberculosis co-infection. Eur. Respir. J. 2010;36(6):1242–1247. doi: 10.1183/09031936.00040910. - DOI - PubMed
LinkOut - more resources
Full Text Sources

