Comparative efficacy of sweated and non-sweated Salvia miltiorrhiza Bge. extracts on acute myocardial ischemia via regulating the PPARα/RXRα/NF-κB signaling pathway
- PMID: 38845919
- PMCID: PMC11154627
- DOI: 10.1016/j.heliyon.2024.e31923
Comparative efficacy of sweated and non-sweated Salvia miltiorrhiza Bge. extracts on acute myocardial ischemia via regulating the PPARα/RXRα/NF-κB signaling pathway
Abstract
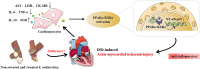
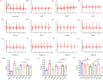
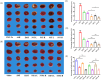
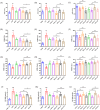
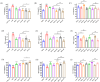
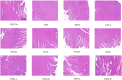
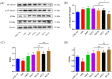
Salvia miltiorrhiza Bge. (S. miltiorrhiza) is a well-known traditional Chinese medicine for the treatment of cardiovascular diseases. The processing of S. miltiorrhiza requires the raw herbs to sweat first and then dry. The aim of this study was to investigate the anti-acute myocardial ischemia (AMI) of S. miltiorrhiza extracts (including tanshinones and phenolic acids) before and after sweating, and to further explore whether the "sweating" primary processing affected the efficacy of S. miltiorrhiza. The AMI animal model was established by subcutaneous injection of isoprenaline hydrochloride (ISO). After treatment, the cardiac function of rats was evaluated by electrocardiogram (ECG), biochemical, and histochemical analysis. Moreover, the regulation of S. miltiorrhiza extracts on the peroxisome proliferator-activated receptor α (PPARα)/retinoid X receptor α (RXRα)/nuclear transcription factor-kappa B (NF-κB) signaling pathway of rats was assessed by the Western blotting. The results showed that sweated and non-sweated S. miltiorrhiza extracts including tanshinones and phenolic acids significantly reduced ST-segment elevation in ECG and the myocardial infarction area in varying degrees. Meanwhile, sweated and non-sweated S. miltiorrhiza reversed the activities of aspartate transaminase (AST), lactic dehydrogenase (LDH), creatine kinase-MB (CK-MB), and superoxide dismutase (SOD), as well as the levels of interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) in AMI rats. Concurrently, the results of Western blotting revealed that S. miltiorrhiza extracts regulated the PPARα/RXRα/NF-κB signaling pathway to exert an anti-inflammatory effect. Most importantly, sweated S. miltiorrhiza tanshinones extracts are more effective than the non-sweated S. miltiorrhiza, and the anti-inflammatory efficacy of tanshinones extract was also better than that of phenolic acid extract. Although phenolic acid extracts before and after sweating were effective in anti-AMI, there was no significant difference between them. In conclusion, both tanshinones and phenolic acids extracts of sweated and non-sweated S. miltiorrhiza promote anti-oxidative stress and anti-inflammatory against AMI via regulating the PPARα/RXRα/NF-κB signaling pathway. Further, the comparations between sweated and non-sweated S. miltiorrhiza extracts indicate that sweated S. miltiorrhiza tanshinones extracts have better therapeutic effects on AMI.
Keywords: Acute myocardial ischemia; Anti-inflammatory; S. miltiorrhiza; Sweating.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










Similar articles
-
Phytochemical profile and protective effects on myocardial ischaemia-reperfusion injury of sweated and non-sweated Salvia miltiorrhiza. Bge alcoholic extracts.J Pharm Pharmacol. 2022 Sep 1;74(9):1230-1240. doi: 10.1093/jpp/rgac012. J Pharm Pharmacol. 2022. PMID: 35833577
-
Comparison of Sweated and Non-Sweated Ethanol Extracts of Salvia miltiorrhiza Bge. (Danshen) Effects on Human and Rat Hepatic UDP-Glucuronosyltransferase and Preclinic Herb-Drug Interaction Potential Evaluation.Curr Drug Metab. 2022;23(6):473-483. doi: 10.2174/1389200223666220517115845. Curr Drug Metab. 2022. PMID: 35585828
-
Salvia miltiorrhiza Extract Prevents the Occurrence of Early Atherosclerosis in Apoe -/- Mice via TLR4/ NF-kB Pathway.Cardiovasc Hematol Agents Med Chem. 2023;21(3):232-239. doi: 10.2174/1871525721666230206112134. Cardiovasc Hematol Agents Med Chem. 2023. PMID: 36748219 Free PMC article.
-
Effects of Salvia miltiorrhiza on CNS Neuronal Injury and Degeneration: A Plausible Complementary Role of Tanshinones and Depsides.Planta Med. 2015 Aug;81(12-13):1003-16. doi: 10.1055/s-0035-1546196. Epub 2015 Jul 17. Planta Med. 2015. PMID: 26190397 Review.
-
The Biosynthetic Pathways of Tanshinones and Phenolic Acids in Salvia miltiorrhiza.Molecules. 2015 Sep 8;20(9):16235-54. doi: 10.3390/molecules200916235. Molecules. 2015. PMID: 26370949 Free PMC article. Review.
Cited by
-
Microbial community variation enhances active compound composition in Salvia miltiorrhiza.Int Microbiol. 2025 Aug 7. doi: 10.1007/s10123-025-00700-4. Online ahead of print. Int Microbiol. 2025. PMID: 40773089
References
-
- Liu H., Yan R., Shao A., Yang B., Ai L. Effect of “sweating” on the quality of Chinese herbal medicines. Chin. J. Exp. Tradit. Med. Formulae. 2013;19:349–352.
-
- Liu Y.Q., Zhang R., Li W., Du H.Z., Wu S.S., Xiong R., Hou X.J., Zhang M., Wang X.J. Study on effects of different habitat processing methods of Salviae Miltiorrhizae Radix et Rhizoma in rats with acute myocardial ischemia. Zhongguo Zhongyao Zazhi. 2020;45(23):5694–5700. doi: 10.19540/j.cnki.cjcmm.20200922.304. - DOI - PubMed
-
- Wang S., Hua Y., Xu L., Zou L., Liu X., Luo Y., Liu J., Yan Y. Quality evaluation of scrophulariae radix processed by different ‘sweating’ methods based on simultaneous determination of multiple bioactive constituents combined with grey relational analysis. Molecules. 2016;21(7):850. doi: 10.3390/molecules21070850. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

