Surface engineering of pure magnesium in medical implant applications
- PMID: 38845950
- PMCID: PMC11153198
- DOI: 10.1016/j.heliyon.2024.e31703
Surface engineering of pure magnesium in medical implant applications
Abstract
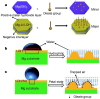
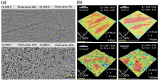
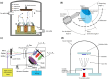
This review comprehensively surveys the latest advancements in surface modification of pure magnesium (Mg) in recent years, with a focus on various cost-effective procedures, comparative analyses, and assessments of outcomes, addressing the merits and drawbacks of pure Mg and its alloys. Diverse economically feasible methods for surface modification, such as hydrothermal processes and ultrasonic micro-arc oxidation (UMAO), are discussed, emphasizing their exceptional performance in enhancing surface properties. The attention is directed towards the biocompatibility and corrosion resistance of pure Mg, underscoring the remarkable efficacy of techniques such as Ca-deficientca-deficient hydroxyapatite (CDHA)/MgF2 bi-layer coating and UMAO coating in electrochemical processes. These methods open up novel avenues for the application of pure Mg in medical implants. Emphasis is placed on the significance of adhering to the principles of reinforcing the foundation and addressing the source. The advocacy is for a judicious approach to corrosion protection on high-purity Mg surfaces, aiming to optimize the overall mechanical performance. Lastly, a call is made for future in-depth investigations into areas such as composite coatings and the biodegradation mechanisms of pure Mg surfaces, aiming to propel the field towards more sustainable and innovative developments.
Keywords: Biocompatibility; Corrosion resistance; Pure magnesium; Surface modification methods.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










Similar articles
-
A Ca-deficientca-deficient hydroxyapatite (CDHA)/MgF2 bi-layer coating with unique nano-scale topography on biodegradable high-purity Mg.Colloids Surf B Biointerfaces. 2020 Jun;190:110911. doi: 10.1016/j.colsurfb.2020.110911. Epub 2020 Feb 27. Colloids Surf B Biointerfaces. 2020. PMID: 32146277
-
Polymer bilayer-Micro arc oxidation surface coating on pure magnesium for bone implantation.J Orthop Translat. 2023 May 24;40:27-36. doi: 10.1016/j.jot.2023.05.003. eCollection 2023 May. J Orthop Translat. 2023. PMID: 37274179 Free PMC article.
-
Preparation and corrosion resistance of magnesium phytic acid/hydroxyapatite composite coatings on biodegradable AZ31 magnesium alloy.J Mater Sci Mater Med. 2017 Jun;28(6):82. doi: 10.1007/s10856-017-5876-9. Epub 2017 Apr 19. J Mater Sci Mater Med. 2017. PMID: 28424946
-
Advances in amelioration of plasma electrolytic oxidation coatings on biodegradable magnesium and alloys.Heliyon. 2024 Jan 11;10(4):e24348. doi: 10.1016/j.heliyon.2024.e24348. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38434039 Free PMC article. Review.
-
Review of the Effect of Surface Coating Modification on Magnesium Alloy Biocompatibility.Materials (Basel). 2022 May 4;15(9):3291. doi: 10.3390/ma15093291. Materials (Basel). 2022. PMID: 35591624 Free PMC article. Review.
Cited by
-
Influence of Noble Metals on Morphology and Topology of Structural Elements in Magnesium Alloy.Materials (Basel). 2024 Aug 23;17(17):4173. doi: 10.3390/ma17174173. Materials (Basel). 2024. PMID: 39274563 Free PMC article.
References
-
- Rupp F., Geis‐Gerstorfer J., Geckeler K.E. Dental implant materials: surface modification and interface phenomena. Adv. Mater. 2010;8 doi: 10.1002/adma.19960080316. - DOI
-
- Yigit O., Gurgenc T., Dikici B., Kaseem M., Boehlert C., Arslan E. Surface modification of pure Mg for enhanced biocompatibility and controlled biodegradation: a study on graphene oxide (GO)/Strontium apatite (SrAp) biocomposite coatings. Coatings. 2023;13 doi: 10.3390/coatings13050890. - DOI
-
- Kassab E., Marquardt A., Neelakantan L., Frotscher M., Schreiber F., Gries T., Jockenhoevel S., Gomes J., Eggeler G. On the electropolishing of NiTi braided stents - challenges and solutions. Materialwiss. Werkstofftech. 2014;45:920–929. doi: 10.1002/mawe.201400220. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

