Treating Type 2 Diabetes With Early, Intensive, Multimodal Pharmacotherapy: Real-World Evidence From an International Collaborative Database
- PMID: 38846063
- PMCID: PMC11156508
- DOI: 10.1155/2024/3470654
Treating Type 2 Diabetes With Early, Intensive, Multimodal Pharmacotherapy: Real-World Evidence From an International Collaborative Database
Abstract
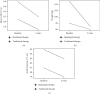
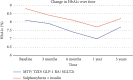
Aims: We compared the glycaemic and cardiorenal effects of combination therapy involving metformin, pioglitazone, sodium-glucose-linked-cotransporter-2 inhibitor (SGLT2i), and glucagon-like peptide-1 receptor agonist (GLP-1RA) versus a more conventional glucocentric treatment approach combining sulphonylureas (SU) and insulin from the point of type 2 diabetes (T2D) diagnosis. Methods: We performed a retrospective cohort study using the Global Collaborative Network in TriNetX. We included individuals prescribed metformin, pioglitazone, an SGLT2i, and a GLP-1 RA for at least 1-year duration, within 3 years of a T2D diagnosis, and compared with individuals prescribed insulin and a SU within the same temporal pattern. Individuals were followed up for 3 years. Results: We propensity score-matched (PSM) for 26 variables. A total of 1762 individuals were included in the final analysis (n = 881 per cohort). At 3-years, compared to the insulin/SU group, the metformin/pioglitazone/SGLT2i/GLP-1 RA group had a lower risk of heart failure (HR 0.34, 95% CI 0.13-0.87, p = 0.018), acute coronary syndrome (HR 0.29, 95% CI 0.12-0.67, p = 0.002), stroke (HR 0.17, 95% CI 0.06-0.49, p < 0.001), chronic kidney disease (HR 0.50, 95% CI 0.25-0.99, p = 0.042), and hospitalisation (HR 0.59, 95% CI 0.46-0.77, p < 0.001). Conclusions: In this real-world study, early, intensive polytherapy, targeting the distinct pathophysiological defects in T2D, is associated with significantly more favourable cardiorenal outcomes, compared to insulin and SU therapy.
Keywords: cardiovascular outcomes; ominous octet; pioglitazone; type 2 diabetes.
Copyright © 2024 Matthew Anson et al.
Conflict of interest statement
M. A. receives a salary from the Pain Relief Foundation. P. A. and G. H. I. are employees of TriNetX LLC. D. J. C. has received investigator-initiated grants from AstraZeneca and Novo Nordisk and support for education from Perspectum. U. A. has received honoraria from Procter & Gamble, Viatris, Grunenthal, and Sanofi for educational meetings. U. A. has also received investigator-led funding from Procter & Gamble. All other authors declare no conflicts of interest.
Figures

References
-
- Saeedi P., Petersohn I., Salpea P., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice . 2019;157, article 107843 doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

