Ferroptosis inhibitors: past, present and future
- PMID: 38846099
- PMCID: PMC11153831
- DOI: 10.3389/fphar.2024.1407335
Ferroptosis inhibitors: past, present and future
Abstract
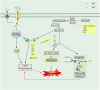
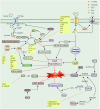
Ferroptosis is a non-apoptotic mode of programmed cell death characterized by iron dependence and lipid peroxidation. Since the ferroptosis was proposed, researchers have revealed the mechanisms of its formation and continue to explore effective inhibitors of ferroptosis in disease. Recent studies have shown a correlation between ferroptosis and the pathological mechanisms of neurodegenerative diseases, as well as diseases involving tissue or organ damage. Acting on ferroptosis-related targets may provide new strategies for the treatment of ferroptosis-mediated diseases. This article specifically describes the metabolic pathways of ferroptosis and summarizes the reported mechanisms of action of natural and synthetic small molecule inhibitors of ferroptosis and their efficacy in disease. The paper also describes ferroptosis treatments such as gene therapy, cell therapy, and nanotechnology, and summarises the challenges encountered in the clinical translation of ferroptosis inhibitors. Finally, the relationship between ferroptosis and other modes of cell death is discussed, hopefully paving the way for future drug design and discovery.
Keywords: antioxidants system; clinical translation; ferroptosis; inhibitors; iron metabolism; lipid metabolism.
Copyright © 2024 Zhang, Luo, Xiang, Bai, Qiang, Zhang, Yang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abdul Y., Li W., Ward R., Abdelsaid M., Hafez S., Dong G., et al. (2021). Deferoxamine treatment prevents post-stroke vasoregression and neurovascular unit remodeling leading to improved functional outcomes in type 2 male diabetic rats: role of endothelial ferroptosis. Transl. Stroke Res. 12, 615–630. 10.1007/s12975-020-00844-7 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

