IDEAL-IQ measurement can distinguish dysplastic nodule from early hepatocellular carcinoma: a case-control study
- PMID: 38846285
- PMCID: PMC11151266
- DOI: 10.21037/qims-23-1593
IDEAL-IQ measurement can distinguish dysplastic nodule from early hepatocellular carcinoma: a case-control study
Abstract
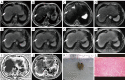
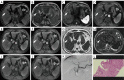
Background: Previous studies have confirmed that malignant transformation of dysplastic nodule (DN) into hepatocellular carcinoma (HCC) is accompanied by reduction of iron content in nodules. This pathological abnormality can serve as the basis for magnetic resonance imaging (MRI). This study was designed to identify the feasibility of iterative decomposition of water and fat with echo asymmetry and least squares estimation-iron quantitative (IDEAL-IQ) measurement to distinguish early hepatocellular carcinoma (eHCC) from DN.
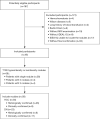
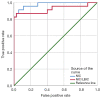
Methods: We reviewed MRI studies of 35 eHCC and 23 DN lesions (46 participants with 58 lesions total, 37 males, 9 females, 31-80 years old). The exams include IDEAL-IQ sequence and 3.0T MR conventional scan [including T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and Gadopentic acid (Gd-GDPA)-enhanced]. Then, 3 readers independently diagnosed eHCC, DN, or were unable to distinguish eHCC from DN using conventional MRI (CMRI), and then assessed R2* value of nodules [R2* value represents the nodule iron content (NIC)] and R2* value of liver background [R2* value represents the liver background iron content (LBIC)] with IDEAL-IQ. Statistical analysis was conducted using the t-test for comparison of means, the Mann-Whitney test for comparison of medians, the chi-square test for comparison of frequencies, and diagnostic efficacy was evaluated by using receiver operating characteristic (ROC) curve.
Results: This study evaluated 35 eHCC participants (17 males, 6 females, 34-81 years old, nodule size: 10.5-27.6 mm, median 18.0 mm) and 23 DN participants (20 males, 3 females, 31-76 years old, nodule size: 16.30±4.095 mm). The NIC and ratio of NIC to LIBC (NIC/LBIC) of the eHCC group (35.926±12.806 sec-1, 0.327±0.107) was lower than that of the DN group (176.635±87.686 sec-1, 1.799±0.629) (P<0.001). Using NIC and NIC/LBIC to distinguish eHCC from DN, the true positive/false positive rates were 91.3%/94.3% and 87.0%/97.1%, respectively. The rates of CMRI, NIC and NIC/LBIC in diagnosis of eHCC were 77.1%, and 94.3%, 97.1%, respectively, and those of DN were 65.2%, 91.3%, and 87.0%, respectively. The diagnosis rate of eHCC and DN by CMRI was lower than that of NIC and NIC/LBIC (eHCC: P=0.03, 0.04, DN: P=0.02, 0.04).
Conclusions: Using IDEAL-IQ measurement can distinguish DN from eHCC.
Keywords: Early hepatocellular carcinoma (eHCC); dysplastic nodule (DN); iterative decomposition of water and fat with echo asymmetry and least squares estimation-iron quantitative (IDEAL-IQ); magnetic resonance imaging (MRI).
2024 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-1593/coif). All authors report that this study received funding from Shenzhen High-level Hospital Construction Fund (No. G2022108). The authors have no other conflicts of interest to declare.
Figures
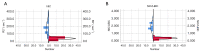
References
LinkOut - more resources
Full Text Sources
