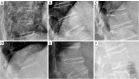
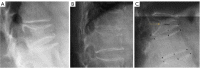
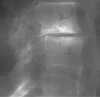
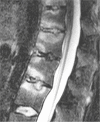
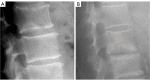
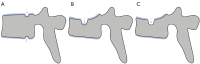
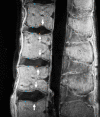
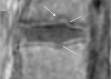
Cartilaginous endplate coverage of developmental Schmorl's node and the relevance of this in Schmorl's node etiology-based classification
- PMID: 38846309
- PMCID: PMC11151241
- DOI: 10.21037/qims-24-335
Cartilaginous endplate coverage of developmental Schmorl's node and the relevance of this in Schmorl's node etiology-based classification
Conflict of interest statement
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-335/coif). Y.X.J.W. serves as the Editor-in-Chief of Quantitative Imaging in Medicine and Surgery. The author has no other conflicts of interest to declare.
Figures
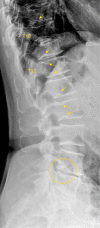

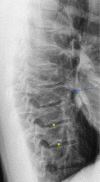
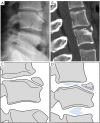
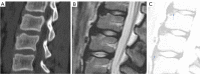
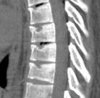
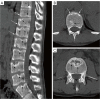

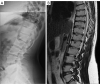
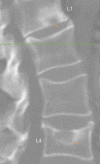


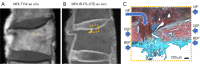
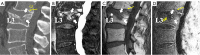
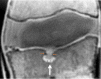
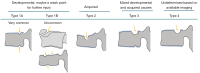
Similar articles
-
Differentiation between spinal subchondral bone metastasis with focal pathologic endplate fracture and oedematous Schmorl's node.J Med Imaging Radiat Oncol. 2022 Oct;66(7):913-919. doi: 10.1111/1754-9485.13365. Epub 2021 Dec 1. J Med Imaging Radiat Oncol. 2022. PMID: 34854219
-
Schmorl's Node: An Uncommon Case of Back Pain and Radiculopathy.Orthop Rev (Pavia). 2022 Apr 25;14(3):33641. doi: 10.52965/001c.33641. eCollection 2022. Orthop Rev (Pavia). 2022. PMID: 35775032 Free PMC article.
-
Schmorl's node may cause an increased FDG activity.Clin Nucl Med. 2011 Jun;36(6):494-5. doi: 10.1097/RLU.0b013e3182173967. Clin Nucl Med. 2011. PMID: 21552037
-
Schmorl's nodes: current pathophysiological, diagnostic, and therapeutic paradigms.Neurosurg Rev. 2014 Jan;37(1):39-46. doi: 10.1007/s10143-013-0488-4. Epub 2013 Aug 18. Neurosurg Rev. 2014. PMID: 23955279 Review.
-
Schmorl's nodes: demystification road of endplate defects-a critical review.Spine Deform. 2022 May;10(3):489-499. doi: 10.1007/s43390-021-00445-w. Epub 2021 Nov 25. Spine Deform. 2022. PMID: 34825353 Review.
Cited by
-
Subtype analysis of Schmorl's nodes in the lumbar spine and the association with lumbar degeneration: a retrospective evaluation of 2262 abdominal CT scans.Eur Spine J. 2025 May;34(5):1722-1730. doi: 10.1007/s00586-025-08827-8. Epub 2025 Apr 7. Eur Spine J. 2025. PMID: 40192771
-
Qualitative and Quantitative MR Imaging of the Cartilaginous Endplate: A Review.J Magn Reson Imaging. 2025 Apr;61(4):1552-1571. doi: 10.1002/jmri.29562. Epub 2024 Aug 20. J Magn Reson Imaging. 2025. PMID: 39165086 Review.
References
-
- Kronthaler S, Boehm C, Feuerriegel G, Börnert P, Katscher U, Weiss K, Makowski MR, Schwaiger BJ, Gersing AS, Karampinos DC. Assessment of vertebral fractures and edema of the thoracolumbar spine based on water-fat and susceptibility-weighted images derived from a single ultra-short echo time scan. Magn Reson Med 2022;87:1771-83. 10.1002/mrm.29078 - DOI - PubMed
LinkOut - more resources
Full Text Sources
