Heterogeneous distribution of mitochondria and succinate dehydrogenase activity in human airway smooth muscle cells
- PMID: 38846375
- PMCID: PMC11150758
- DOI: 10.1096/fba.2024-00047
Heterogeneous distribution of mitochondria and succinate dehydrogenase activity in human airway smooth muscle cells
Abstract
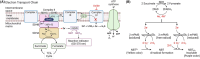
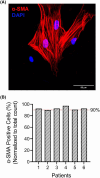
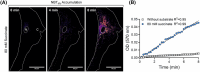
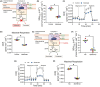
Succinate dehydrogenase (SDH) is a key mitochondrial enzyme involved in the tricarboxylic acid cycle, where it facilitates the oxidation of succinate to fumarate, and is coupled to the reduction of ubiquinone in the electron transport chain as Complex II. Previously, we developed a confocal-based quantitative histochemical technique to determine the maximum velocity of the SDH reaction (SDHmax) in single cells and observed that SDHmax corresponds with mitochondrial volume density. In addition, mitochondrial volume and motility varied within different compartments of human airway smooth muscle (hASM) cells. Therefore, we hypothesize that the SDH activity varies relative to the intracellular mitochondrial volume within hASM cells. Using 3D confocal imaging of labeled mitochondria and a concentric shell method for analysis, we quantified mitochondrial volume density, mitochondrial complexity index, and SDHmax relative to the distance from the nuclear membrane. The mitochondria within individual hASM cells were more filamentous in the immediate perinuclear region and were more fragmented in the distal parts of the cell. Within each shell, SDHmax also corresponded to mitochondrial volume density, where both peaked in the perinuclear region and decreased in more distal parts of the cell. Additionally, when normalized to mitochondrial volume, SDHmax was lower in the perinuclear region when compared to the distal parts of the cell. In summary, our results demonstrate that SDHmax measures differences in SDH activity within different cellular compartments. Importantly, our data indicate that mitochondria within individual cells are morphologically heterogeneous, and their distribution varies substantially within different cellular compartments, with distinct functional properties.
Keywords: airway smooth muscle; confocal microscope; intracellular distribution; mitochondria; succinate dehydrogenase.
© 2024 The Author(s). FASEB BioAdvances published by Wiley Periodicals LLC on behalf of The Federation of American Societies for Experimental Biology.
Figures
Similar articles
-
TNFα-mediated subcellular heterogeneity of succinate dehydrogenase activity in human airway smooth muscle cells.Am J Physiol Lung Cell Mol Physiol. 2025 Jun 1;328(6):L792-L808. doi: 10.1152/ajplung.00396.2024. Epub 2025 Apr 23. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 40266735 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Does Augmenting Irradiated Autografts With Free Vascularized Fibula Graft in Patients With Bone Loss From a Malignant Tumor Achieve Union, Function, and Complication Rate Comparably to Patients Without Bone Loss and Augmentation When Reconstructing Intercalary Resections in the Lower Extremity?Clin Orthop Relat Res. 2025 Jun 26;483(9):1680-95. doi: 10.1097/CORR.0000000000003599. Online ahead of print. Clin Orthop Relat Res. 2025. PMID: 40569278
-
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD011551. doi: 10.1002/14651858.CD011551.pub2. Cochrane Database Syst Rev. 2018. PMID: 29357120 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
Cited by
-
TNFα-mediated subcellular heterogeneity of succinate dehydrogenase activity in human airway smooth muscle cells.Am J Physiol Lung Cell Mol Physiol. 2025 Jun 1;328(6):L792-L808. doi: 10.1152/ajplung.00396.2024. Epub 2025 Apr 23. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 40266735 Free PMC article.
References
-
- Cecchini G. Function and structure of complex II of the respiratory chain. Annu Rev Biochem. 2003;72:77‐109. - PubMed
-
- Rustin P, Munnich A, Rotig A. Succinate dehydrogenase and human diseases: new insights into a well‐known enzyme. Eur J Hum Genet. 2002;10:289‐291. - PubMed
-
- Sieck GC, Sacks RD, Blanco CE, Edgerton VR. SDH activity and cross‐sectional area of muscle fibers in cat diaphragm. J Appl Physiol (1985). 1986;60:1284‐1292. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
