The effects of oxidative stress and intracellular calcium on mitochondrial permeability transition pore formation in equine spermatozoa
- PMID: 38846376
- PMCID: PMC11150759
- DOI: 10.1096/fba.2023-00051
The effects of oxidative stress and intracellular calcium on mitochondrial permeability transition pore formation in equine spermatozoa
Abstract
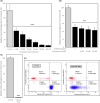
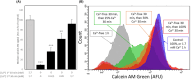
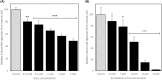
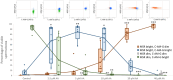
The in vitro storage of stallion spermatozoa for use in artificial insemination leads to oxidative stress and imbalances in calcium homeostasis that trigger the formation of the mitochondrial permeability transition pore (mPTP), resulting in premature cell death. However, little is understood about the dynamics and the role of mPTP formation in mammalian spermatozoa. Here, we identify an important role for mPTP in stallion sperm Ca2+ homeostasis. We show that stallion spermatozoa do not exhibit "classical" features of mPTP; specifically, they are resistant to cyclosporin A-mediated inhibition of mPTP formation, and they do not require exogenous Ca2+ to form the mPTP. However, chelation of endogenous Ca2+ prevented mPTP formation, indicating a role for intracellular Ca2+ in this process. Furthermore, our findings suggest that this cell type can mobilize intracellular Ca2+ stores to form the mPTP in response to low Ca2+ environments and that under oxidative stress conditions, mPTP formation preceded a measurable increase in intracellular Ca2+, and vice versa. Contrary to previous work that identified mitochondrial membrane potential (MMP) as a proxy for mPTP formation, here we show that a loss of MMP can occur independently of mPTP formation, and thus MMP is not an appropriate proxy for the detection of mPTP formation. In conclusion, the mPTP plays a crucial role in maintaining Ca2+ and reactive oxygen species homeostasis in stallion spermatozoa, serving as an important regulatory mechanism for normal sperm function, thereby contraindicating the in vitro pharmacological inhibition of mPTP formation to enhance sperm longevity.
Keywords: JC‐1; horse; mitochondrial permeability transition pore; oxidative stress; spermatozoa.
© 2024 The Authors. FASEB BioAdvances published by Wiley Periodicals LLC on behalf of The Federation of American Societies for Experimental Biology.
Figures



Similar articles
-
Exogenous Oxidative Stress in Human Spermatozoa Induces Opening of the Mitochondrial Permeability Transition Pore: Effect on Mitochondrial Function, Sperm Motility and Induction of Cell Death.Antioxidants (Basel). 2024 Jun 18;13(6):739. doi: 10.3390/antiox13060739. Antioxidants (Basel). 2024. PMID: 38929178 Free PMC article.
-
Mitochondrial permeability transition pore (MPTP) desensitization increases sea urchin spermatozoa fertilization rate.Cell Biol Int. 2016 Oct;40(10):1071-83. doi: 10.1002/cbin.10647. Epub 2016 Aug 3. Cell Biol Int. 2016. PMID: 27449751
-
The Ca²⁺-calmodulin-Ca²⁺/calmodulin-dependent protein kinase II signaling pathway is involved in oxidative stress-induced mitochondrial permeability transition and apoptosis in isolated rat hepatocytes.Arch Toxicol. 2014 Sep;88(9):1695-709. doi: 10.1007/s00204-014-1219-5. Epub 2014 Mar 11. Arch Toxicol. 2014. PMID: 24614978
-
The Mitochondrial Permeability Transition Pore-Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State.Cells. 2023 Apr 27;12(9):1273. doi: 10.3390/cells12091273. Cells. 2023. PMID: 37174672 Free PMC article. Review.
-
Mitochondrial Permeability Transition in Stem Cells, Development, and Disease.Adv Exp Med Biol. 2023;1409:1-22. doi: 10.1007/5584_2022_720. Adv Exp Med Biol. 2023. PMID: 35739412 Review.
References
-
- Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011;14:367‐381. - PubMed
-
- Gibb Z, Lambourne SR, Curry BJ, Hall SE, Aitken RJ. Aldehyde dehydrogenase plays a pivotal role in the maintenance of stallion sperm motility. Biol Reprod. 2016;94(6):1‐11. - PubMed
-
- White IG. Lipids and calcium uptake of sperm in relation to cold shock and preservation: a review. Reprod Fertil Dev. 1993;5:639‐658. - PubMed
-
- Gibb Z, Holt B, Swegen A, Lambourne SR, Aitken RJ. Mitochondrial permeability transition pore formation during chilling and cryopreservation of stallion spermatozoa. Paper Presented at: 48th Annual Scientific Meeting of the Endocrine Society of Australia and the Society for Reproductive Biology; August 27–30, 2017; Perth, Australia.
-
- Stewart TA, Davis FM. An element for development: calcium signaling in mammalian reproduction and development. Biochim Biophys Acta Mol Cell Res. 2019;1866:1230‐1238. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
