Radical ligand transfer: mechanism and reactivity governed by three-component thermodynamics
- PMID: 38846394
- PMCID: PMC11151871
- DOI: 10.1039/d4sc01507j
Radical ligand transfer: mechanism and reactivity governed by three-component thermodynamics
Erratum in
-
Correction: Radical ligand transfer: mechanism and reactivity governed by three-component thermodynamics.Chem Sci. 2025 Jan 7;16(4):2046. doi: 10.1039/d4sc90254h. eCollection 2025 Jan 22. Chem Sci. 2025. PMID: 39781221 Free PMC article.
Abstract
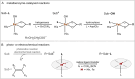
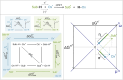
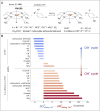
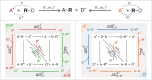
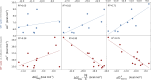
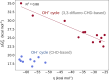
Here, we demonstrate that the relationship between reactivity and thermodynamics in radical ligand transfer chemistry can be understood if this chemistry is dissected as concerted ion-electron transfer (cIET). Namely, we investigate radical ligand transfer reactions from the perspective of thermodynamic contributions to the reaction barrier: the diagonal effect of the free energy of the reaction, and the off-diagonal effect resulting from asynchronicity and frustration, which we originally derived from the thermodynamic cycle for concerted proton-electron transfer (cPET). This study on the OH transfer reaction shows that the three-component thermodynamic model goes beyond cPET chemistry, successfully capturing the changes in radical ligand transfer reactivity in a series of model FeIII-OH⋯(diflouro)cyclohexadienyl systems. We also reveal the decisive role of the off-diagonal thermodynamics in determining the reaction mechanism. Two possible OH transfer mechanisms, in which electron transfer is coupled with either OH- and OH+ transfer, are associated with two competing thermodynamic cycles. Consequently, the operative mechanism is dictated by the cycle yielding a more favorable off-diagonal effect on the barrier. In line with this thermodynamic link to the mechanism, the transferred OH group in OH-/electron transfer retains its anionic character and slightly changes its volume in going from the reactant to the transition state. In contrast, OH+/electron transfer develops an electron deficiency on OH, which is evidenced by an increase in charge and a simultaneous decrease in volume. In addition, the observations in the study suggest that an OH+/electron transfer reaction can be classified as an adiabatic radical transfer, and the OH-/electron transfer reaction as a less adiabatic ion-coupled electron transfer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Elucidation of factors shaping reactivity of 5'-deoxyadenosyl - a prominent organic radical in biology.Phys Chem Chem Phys. 2024 Jul 31;26(30):20280-20295. doi: 10.1039/d4cp01725k. Phys Chem Chem Phys. 2024. PMID: 39041228
-
A Continuum of Proton-Coupled Electron Transfer Reactivity.Acc Chem Res. 2018 Oct 16;51(10):2391-2399. doi: 10.1021/acs.accounts.8b00319. Epub 2018 Sep 20. Acc Chem Res. 2018. PMID: 30234963 Free PMC article.
-
Separating Proton and Electron Transfer Effects in Three-Component Concerted Proton-Coupled Electron Transfer Reactions.J Am Chem Soc. 2017 Aug 2;139(30):10312-10319. doi: 10.1021/jacs.7b03562. Epub 2017 Jul 21. J Am Chem Soc. 2017. PMID: 28671470 Free PMC article.
-
Ion cyclotron resonance spectroscopy. Cyclotron double resonance provides a new technique for the study of ion-molecule reaction mechanisms.Science. 1968 Jan 19;159(3812):263-73. doi: 10.1126/science.159.3812.263. Science. 1968. PMID: 4863791 Review.
-
Food Antioxidants: Chemical Insights at the Molecular Level.Annu Rev Food Sci Technol. 2016;7:335-52. doi: 10.1146/annurev-food-041715-033206. Epub 2016 Jan 11. Annu Rev Food Sci Technol. 2016. PMID: 26772412 Review.
References
-
- Lloyd M. D. Merritt K. D. Lee V. Sewell T. J. Wha-Son B. Baldwin J. E. Schofield C. J. Elson S. W. Baggaley K. H. Nicholson N. H. Tetrahedron. 1999;55:10201–10220. doi: 10.1016/S0040-4020(99)00547-5. - DOI
LinkOut - more resources
Full Text Sources

