High Strength and Shape Memory Spinal Fusion Device for Minimally Invasive Interbody Fusions
- PMID: 38846643
- PMCID: PMC11155384
- DOI: 10.2147/IJN.S460339
High Strength and Shape Memory Spinal Fusion Device for Minimally Invasive Interbody Fusions
Abstract
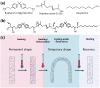
Introduction: Lumbar interbody fusion is widely employed for both acute and chronic spinal diseases interventions. However, large incision created during interbody cage implantation may adversely impair spinal tissue and influence postoperative recovery. The aim of this study was to design a shape memory interbody fusion device suitable for small incision implantation.
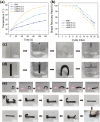
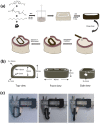
Methods: In this study, we designed and fabricated an intervertebral fusion cage that utilizes near-infrared (NIR) light-responsive shape memory characteristics. This cage was composed of bisphenol A diglycidyl ether, polyether amine D-230, decylamine and iron oxide nanoparticles. A self-hardening calcium phosphate-starch cement (CSC) was injected internally through the injection channel of the cage for healing outcome improvement.
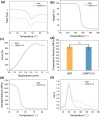
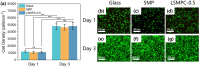
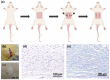
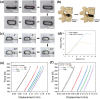
Results: The size of the interbody cage is reduced from 22 mm to 8.8 mm to minimize the incision size. Subsequent NIR light irradiation prompted a swift recovery of the cage shape within 5 min at the lesion site. The biocompatibility of the shape memory composite was validated through in vitro MC3T3-E1 cell (osteoblast-like cells) adhesion and proliferation assays and subcutaneous implantation experiments in rats. CSC was injected into the cage, and the relevant results revealed that CSC is uniformly dispersed within the internal space, along with the cage compressive strength increasing from 12 to 20 MPa.
Conclusion: The results from this study thus demonstrated that this integrated approach of using a minimally invasive NIR shape memory spinal fusion cage with CSC has potential for lumbar interbody fusion.
Keywords: NIR responsive; calcium phosphate cement; interbody fusion cage; minimally invasive; shape memory.
© 2024 Liu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Minimally invasive transforaminal lumbar interbody fusion with expandable versus static interbody devices: radiographic assessment of sagittal segmental and pelvic parameters.Neurosurg Focus. 2017 Aug;43(2):E10. doi: 10.3171/2017.5.FOCUS17197. Neurosurg Focus. 2017. PMID: 28760032
-
Minimally Invasive Transforaminal Lumbar Interbody Fusion Using Expandable Cages: Increased Risk of Late Postoperative Subsidence Without a Real Improvement of Perioperative Outcomes: A Clinical Monocentric Study.World Neurosurg. 2021 Dec;156:e57-e63. doi: 10.1016/j.wneu.2021.08.127. Epub 2021 Sep 4. World Neurosurg. 2021. PMID: 34492389
-
Facet Joint Fixation and Anterior, Direct Lateral, and Transforaminal Lumbar Interbody Fusions for Treatment of Degenerative Lumbar Disc Diseases: Retrospective Cohort Study of a New Minimally Invasive Technique.World Neurosurg. 2018 Jun;114:e959-e968. doi: 10.1016/j.wneu.2018.03.121. Epub 2018 Mar 26. World Neurosurg. 2018. PMID: 29588241
-
Device solutions for a challenging spine surgery: minimally invasive transforaminal lumbar interbody fusion (MIS TLIF).Expert Rev Med Devices. 2019 Apr;16(4):299-305. doi: 10.1080/17434440.2019.1601013. Epub 2019 Apr 3. Expert Rev Med Devices. 2019. PMID: 30917071 Review.
-
Assessing the Difference in Clinical and Radiologic Outcomes Between Expandable Cage and Nonexpandable Cage Among Patients Undergoing Minimally Invasive Transforaminal Interbody Fusion: A Systematic Review and Meta-Analysis.World Neurosurg. 2019 Jul;127:596-606.e1. doi: 10.1016/j.wneu.2019.03.284. Epub 2019 Apr 5. World Neurosurg. 2019. PMID: 30954733
References
-
- Delaey J, Dubruel P, Van Vlierberghe S. Shape-Memory Polymers for Biomedical Applications. Adv Funct Mater. 2020;30(44):1909047. doi:10.1002/adfm.201909047 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

