Crafting Docetaxel-Loaded Albumin Nanoparticles Through a Novel Thermal-Driven Self-Assembly/Microfluidic Combination Technology: Formulation, Process Optimization, Stability, and Bioavailability
- PMID: 38846644
- PMCID: PMC11155381
- DOI: 10.2147/IJN.S457482
Crafting Docetaxel-Loaded Albumin Nanoparticles Through a Novel Thermal-Driven Self-Assembly/Microfluidic Combination Technology: Formulation, Process Optimization, Stability, and Bioavailability
Abstract
Background: The commercial docetaxel (DTX) formulation causes severe side effects due to polysorbate 80 and ethanol. Novel surfactant-free nanoparticle (NP) systems are needed to improve bioavailability and reduce side effects. However, controlling the particle size and stability of NPs and improving the batch-to-batch variation are the major challenges.
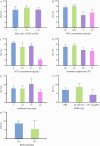
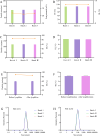
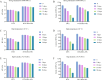
Methods: DTX-loaded bovine serum albumin nanoparticles (DTX-BSA-NPs) were prepared by a novel thermal-driven self-assembly/microfluidic technology. Single-factor analysis and orthogonal test were conducted to obtain the optimal formulation of DTX-BSA-NPs in terms of particle size, encapsulation efficiency (EE), and drug loading (DL). The effects of oil/water flow rate and pump pressure on the particle size, EE, and DL were investigated to optimize the preparation process of DTX-BSA-NPs. The drug release, physicochemical properties, stability, and pharmacokinetics of NPs were evaluated.
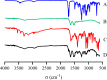
Results: The optimized DTX-BSA-NPs were uniform, with a particle size of 118.30 nm, EE of 89.04%, and DL of 8.27%. They showed a sustained release of 70% over 96 hours and an increased stability. There were some interactions between the drug and excipients in DTX-BSA-NPs. The half-life, mean residence time, and area under the curve (AUC) of DTX-BSA-NPs increased, but plasma clearance decreased when compared with DTX.
Conclusion: The thermal-driven self-assembly/microfluidic combination method effectively produces BSA-based NPs that improve the bioavailability and stability of DTX, offering a promising alternative to traditional formulations.
Keywords: DTX-BSA nanoparticles; in-vitro release; microfluidic technology; pharmacokinetics; thermal-driven self-assembly.
© 2024 Du et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures










References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

