Effect of oral tryptamines on the gut microbiome of rats-a preliminary study
- PMID: 38846751
- PMCID: PMC11155674
- DOI: 10.7717/peerj.17517
Effect of oral tryptamines on the gut microbiome of rats-a preliminary study
Abstract
Background: Psilocybin and related tryptamines have come into the spotlight in recent years as potential therapeutics for depression. Research on the mechanisms of these effects has historically focused on the direct effects of these drugs on neural processes. However, in addition to such neural effects, alterations in peripheral physiology may also contribute to their therapeutic effects. In particular, substantial support exists for a gut microbiome-mediated pathway for the antidepressant efficacy of other drug classes, but no prior studies have determined the effects of tryptamines on microbiota.
Methods: To address this gap, in this preliminary study, male Long Evans rats were treated with varying dosages of oral psilocybin (0.2 or 2 mg/kg), norbaeocystin (0.25 or 2.52 mg/kg), or vehicle and their fecal samples were collected 1 week and 3 weeks after exposure for microbiome analysis using integrated 16S ribosomal DNA sequencing to determine gut microbiome composition.
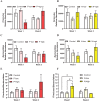
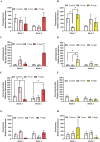
Results: We found that although treatment with neither psilocybin nor norbaeocystin significantly affected overall microbiome diversity, it did cause significant dose- and time-dependent changes in bacterial abundance at the phylum level, including increases in Verrucomicrobia and Actinobacteria, and decreases in Proteobacteria.
Conclusion and implications: These preliminary findings support the idea that psilocybin and other tryptamines may act on the gut microbiome in a dose- and time-dependent manner, potentially identifying a novel peripheral mechanism for their antidepressant activity. The results from this preliminary study also suggest that norbaeocystin may warrant further investigation as a potential antidepressant, given the similarity of its effects to psilocybin.
Keywords: Actinobacteria; Gut microbiome; Norbaeocystin; Proteobacteria; Psilocybin; Rat; Verrucomicrobia.
© 2024 Xu et al.
Conflict of interest statement
J. Andrew Jones is a significant stakeholder at PsyBio Therapeutics. PsyBio Therapeutics has licensed tryptamine biosynthesis-related technology from Miami University. J. Andrew Jones and Matthew S. McMurray are co-inventors on several patent applications related to tryptamine biosynthesis and the impacts of tryptamines on animal behavior. All other authors declare no conflicts of interest.
Figures

References
-
- Adams AM, Anas NA, Sen AK, Hinegardner-Hendricks JD, O’Dell PJ, Gibbons WJ, Jr., Flower JE, McMurray MS, Jones JA. Development of an E. coli-based norbaeocystin production platform and evaluation of behavioral effects in rats. Metabolic Engineering Communications. 2022;14:e00196. doi: 10.1016/j.mec.2022.e00196. - DOI - PMC - PubMed
-
- Anderson JR, Carroll I, Azcarate-Peril MA, Rochette AD, Heinberg LJ, Peat C, Steffen K, Manderino LM, Mitchell J, Gunstad J. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Medicine. 2017;38(6315):104–107. doi: 10.1016/j.sleep.2017.07.018. - DOI - PMC - PubMed
-
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Kristiansen K, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M’Rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P, Meta HITC. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

